[ad_1]
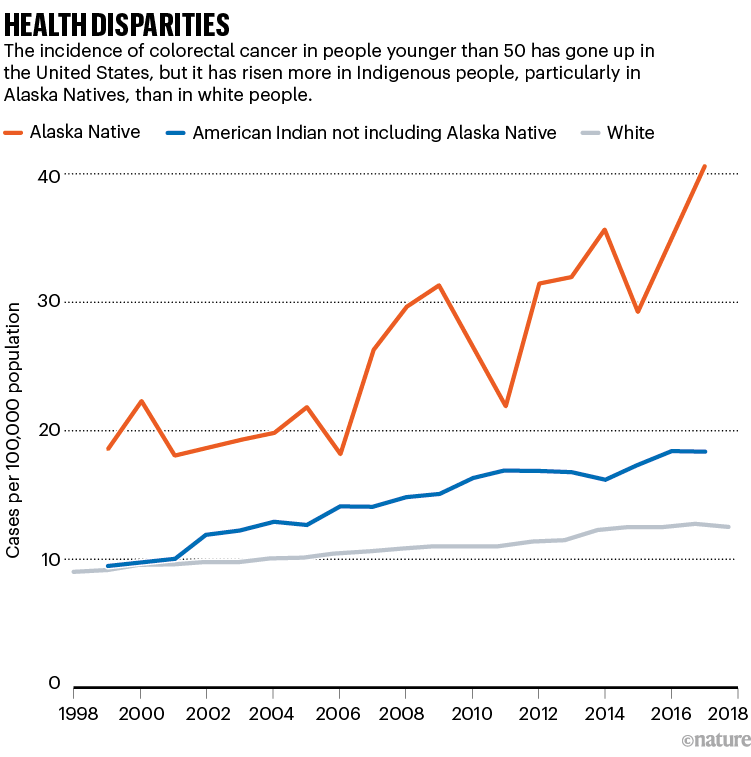
US drug regulators dropped a bombshell in November 2023 when they announced an investigation into one of the most celebrated cancer treatments to emerge in decades. The US Food and Drug Administration (FDA) said it was looking at whether a strategy that involves engineering a person’s immune cells to kill cancer was leading to new malignancies in people who had been treated with it.
Bruce Levine, an immunologist at the University of Pennsylvania Perelman School of Medicine in Philadelphia who helped to pioneer the approach known as chimeric antigen receptor (CAR) T-cell therapy, says he didn’t hear the news until a reporter asked him for comments on the FDA’s announcement.
“Better get smart about it quick,” he remembers thinking.
Although the information provided by the FDA was thin at the time, the agency told reporters that it had observed 20 cases in which immune-cell cancers known as lymphomas had developed in people treated with CAR T cells. Levine, who is a co-inventor of Kymriah, the first CAR-T-cell therapy to be approved, started jotting down questions. Who were these patients? How many were there? And what other drugs had they received before having CAR-T-cell therapy?
How to supercharge cancer-fighting cells: give them stem cell skills
The FDA has since documented more cases. As of 25 March, the agency had received 33 reports of such lymphomas among some 30,000 people who had been treated. It now requires all CAR-T therapies to carry a boxed warning on the drug’s packaging, which states that such cancers have occurred. And the European Medicines Agency has launched its own investigation. But many of the questions that Levine had in November remain unanswered. It is unclear how many, if any, of the observed cancers came directly from the manipulations made to the CAR T cells. A lot of cancer therapies carry a risk of causing secondary malignancies, and the treated individuals had received other therapies. As Crystal Mackall, a paediatric oncologist who heads the cancer immunotherapy programme at Stanford University in California, puts it: “Do you have a smoking gun?”
Scientists are now racing to determine whether the cellular therapy is driving these cancers or contributing in some way to their development. From the data available so far, the secondary cancers seem to be a rare phenomenon, and the benefits of CAR T cells still outweigh the risks for most prospective recipients. But it’s an important puzzle to solve so that researchers can improve and expand the use of these engineered cells in medicine. CAR-T-cell treatments were once reserved for people who had few other options for therapy. But the FDA has approved several of these treatments as a relatively early, second-line option for lymphoma and multiple myeloma. And some companies are working to expand the therapy’s repertoire to solid tumours, autoimmune diseases, ageing, HIV and more.
Aric Hall, a haematologist at the University of Wisconsin–Madison, says that despite the enthusiasm for CAR-T therapy, the technology is still new. “I used to joke that for the first ten years there were more review articles about CAR T than there were patients who had been treated by CAR T products,” he says. He adds that the risks might be rare, but as CAR-T therapy moves into a bigger pool of patients who aren’t desperately ill, the calculus could change. “The problem is rare risks become a bigger deal when patients have better options.”
Vector safety
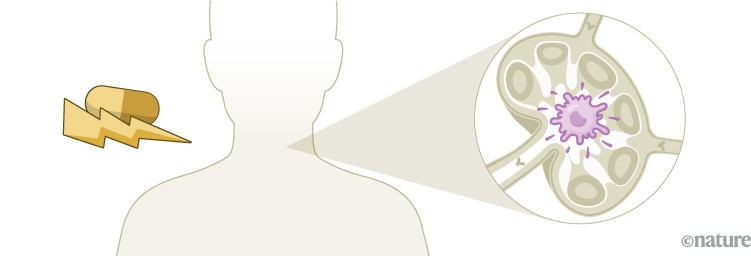
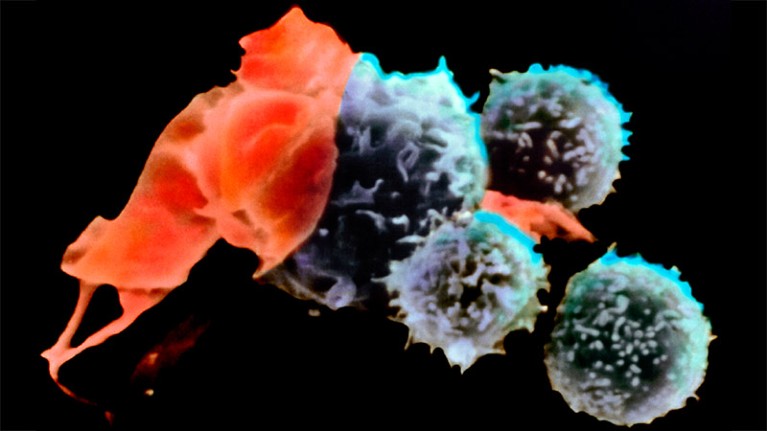
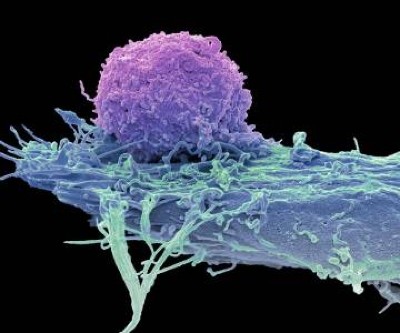
Throughout the development of these blockbuster therapies, researchers had reason to think that CAR T cells could become cancerous. CAR-T therapies are personalized — created from a person’s immune cells. Their T cells are extracted and then genetically modified in the laboratory to express a chimeric antigen receptor — or CAR — a protein that targets the T cell to specific cells that they will kill. T cells sporting these receptors are made to multiply and grow in the lab, and physicians then infuse them back into the individual, where they start battling cancer cells. The six CAR-T-cell therapies currently approved in the United States and Europe target antigens on the immune system’s B cells, so they work only against B-cell malignancies — leukaemias, lymphomas and multiple myeloma. But researchers are aiming to develop CAR-T therapies that work on other kinds of cancer, and for other conditions.
The genetic engineering is the step that creates a risk of malignancy. All six FDA-approved CAR-T therapies rely on a retrovirus — typically a lentivirus, such as HIV, or a gammaretrovirus — to ferry the genetic information into the cell. Scientists remove the parts of the viral genome that allow the virus to replicate, making room for the gene they want the virus vector to carry. Once inside a cell, the virus inserts the gene for the CAR into the cell’s genome. But there isn’t a good way to control exactly where the gene goes. If it slips in near a gene that can promote cancer development and activates it, or if it deactivates a tumour-suppressing gene, that boosts the risk of causing a T-cell cancer (see ‘CAR-T concerns’).
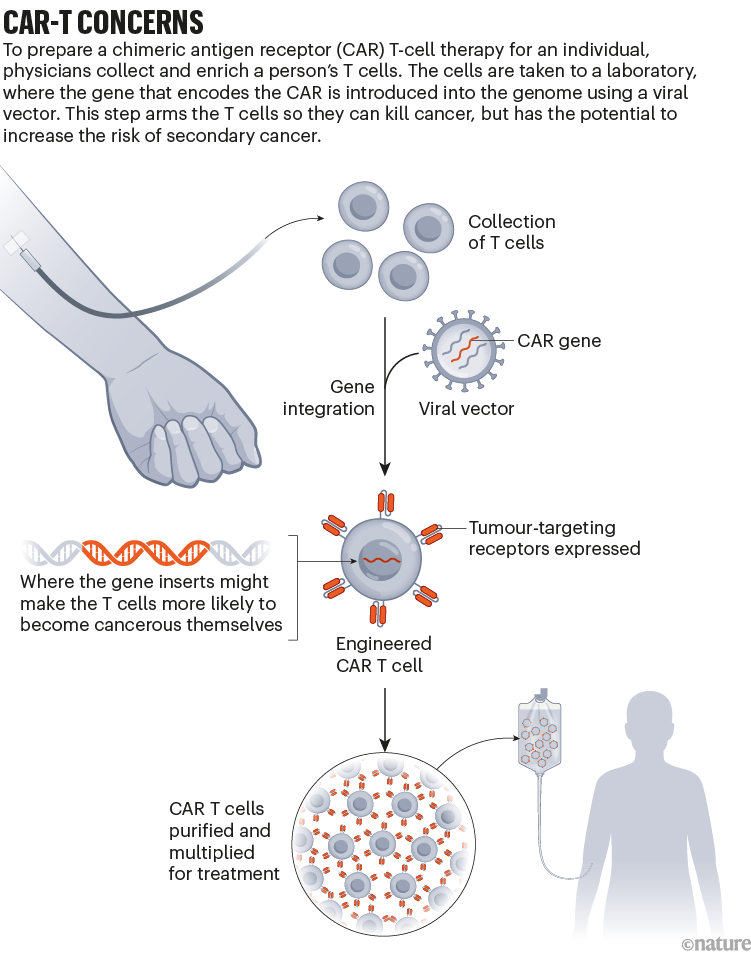
This phenomenon, known as insertional mutagenesis, is a risk with most gene therapies. About 20 years ago, for example, groups in London and Paris treated 20 infants who had severe combined immunodeficiency syndrome (SCID) with a gene therapy that used a retrovirus. The therapy worked for most participants, but the retrovirus switched on cancer genes in some. That activation led to leukaemia in five of the participants; four recovered and one died.
As a result, scientists have reworked the vectors to make them safer, ensuring that their genes don’t recombine, for example. The FDA recommends that CAR-T products undergo testing to prove that the vectors cannot replicate. “The scrutiny we’ve been under has been tremendous,” says Hans-Peter Kiem, an oncologist at the Fred Hutchinson Cancer Center in Seattle, Washington, who has studied viral vectors for decades. Many felt confident about using viral vectors in CAR-T therapies, because T cells are difficult to prod towards malignancy, says Marco Ruella, a haematologist at the Perelman School of Medicine. “Truly the general feeling was that, in T cells, lenti- and retroviruses are extremely safe.”
Search for the smoking gun
When the FDA issued its warning in November, it wasn’t clear what specific reports had prompted the agency to act, or whether the link was causal. Levine recruited some of the biggest names in CAR-T therapies to co-write a commentary on the matter and discuss some of the questions he still had1. “I felt — we felt — that it was important to say, ‘Well, let’s take a step back for a minute and see what we really know,’” he says.
Turbocharged CAR-T cells melt tumours in mice — using a trick from cancer cells
In January, the FDA released more information. In an article in the New England Journal of Medicine, Peter Marks and Nicole Verdun at the FDA’s Center for Biologics Evaluation and Research in Silver Spring, Maryland, revealed that the agency had received 22 reports of leukaemia out of more than 27,000 people treated with various CAR-T therapies2. In three secondary cancers that were sequenced, the agency found that the cancerous T cells contained the CAR gene, “which indicates that the CAR-T product was most likely involved in the development of the T-cell cancer”, the authors wrote.
According to Paul Richards, a spokesperson for the FDA, 11 further reports of secondary cancer have since come in, as of 25 March. None of the extra cases has been confirmed as having the CAR gene, but neither are any of the cases so far definitively CAR-negative, Richards said in an e-mail. In many instances, the agency didn’t have a sample of the secondary cancer to analyse; in others, the genomic analysis isn’t yet complete. He adds that certain reports, specifically those positive for the CAR gene, “strongly suggest” that T-cell cancer should be considered a risk of the therapy.
But even when the CAR gene is present, proving causality can be tricky. In one case study, researchers in Australia described3 a 51-year-old man who had been treated for multiple myeloma with a CAR-T therapy. The treatment was part of a clinical trial of Carvykti, made by Legend Biotech in Somerset, New Jersey, in partnership with the drug giant Johnson & Johnson. The treatment worked to clear his cancer, but five months later he developed an unusual, fast-growing bump on his nose. A biopsy revealed that it was T-cell lymphoma. When the team examined the cancerous cells, they found the gene for the CAR wedged into the regulatory region of a gene called PBX2.
Cutting-edge CAR-T cancer therapy is now made in India — at one-tenth the cost
The finding is provocative, Mackall says, but still not a smoking gun, in her opinion. The researchers found that the cancer cells also carried a mutation often seen in lymphomas, and the person had a genetic variant that put him at increased risk of developing cancer, even without the CAR insertion. It’s likely that the cells harvested to create the therapy contained some pre-cancerous T cells, says Piers Blombery, a haematologist at the Peter MacCallum Cancer Centre in Melbourne, Australia, who leads the diagnostic lab that assessed the tumour samples. Now, the team is looking at samples taken before the therapy to determine whether that’s the case.
Other people who have received Carvykti have developed secondary cancers, too. The FDA’s initial warning focused on T-cell malignancies. But long-term follow-up of participants who’d been in an early trial of Carvykti revealed that 10 out of 97 people developed either myelodysplastic syndrome (a kind of pre-leukaemia) or acute myeloid leukaemia (see go.nature.com/3q8vrym). Nine of them died. As a result, in December 2023, Legend Biotech added language to its boxed warning for Carvykti about the risk of secondary blood cancers.
Craig Tendler, head of oncology clinical development and global medical affairs at Johnson & Johnson Innovative Medicine in Raritan, New Jersey, says that the company looked for the CAR gene in cancer cells from these individuals, but didn’t find it. When the researchers looked at samples taken before the trial participants received treatment, they found pre-malignant cells with the same genetic make-up as the cancer cells. “So, it is likely that, in many of these cases, the prior therapies for multiple myeloma may have already predisposed these patients to secondary malignancy,” Tendler says. Then, it’s possible that the prolonged immune suppression related to the CAR-T treatment process nudged the cells to become cancerous.
Can autoimmune diseases be cured? Scientists see hope at last
When Ruella first saw the FDA warning, he immediately thought back to a 64-year-old man he had treated who, in 2020, developed a T-cell lymphoma 3 months after receiving CAR-T therapy for a B-cell lymphoma. Ruella and his colleagues identified the CAR gene in the biopsy taken from the man’s lymph node4. But it was at such low levels that it seemed unlikely it had integrated into the cancer cells, Ruella says. Instead, the genes could have come from CAR T cells that just happened to be circulating through that lymph node at the time the biopsy was taken. “We thought this was just an accidental finding,” Ruella says.
But after Ruella saw the FDA’s warning, he decided to revisit the case. He and his colleagues went back to a blood sample taken before the person received CAR-T therapy. The team assessed whether T cells with the same T-cell receptor as the lymphoma cells were present before treatment. They were, suggesting that the seeds of the lymphoma pre-dated the therapy. (The low number of cells made further analysis difficult.) Ruella adds that it’s possible the CAR-T treatment produced an inflammatory environment that allowed such seeds to become cancerous. “So this is not something that appears magically out of nowhere,” Ruella says.
Rare outcome
The good news is that these secondary cancers — CAR-driven or not — seem to be rare. After the FDA warning, Ruella and his colleagues also looked back at the files of people who had been treated with commercial CAR-T products at the University of Pennsylvania. Between January 2018 and November 2023, the centre treated 449 individuals who had leukaemias, lymphomas or multiple myeloma with CAR-T therapies4. Sixteen patients (3.6%) went on to develop a secondary cancer. But most of those were solid tumours, not the kind of cancer one would expect to come directly from the treatment. Only five of the treated patients developed blood cancers, and only one of those developed a T-cell cancer.
At the Mayo Clinic in Phoenix, Arizona, haematologist Rafael Fonseca and his colleagues also wondered whether the incidence of secondary cancers in people who had received CAR-T therapy differed from the incidence in those with the same cancers who had not. They combed through a data set containing medical records from 330 million people to find individuals who had been newly diagnosed with multiple myeloma or diffuse large B-cell lymphoma between 2018 and 2022. They then looked at how many of them developed T-cell lymphomas. The prevalence didn’t differ drastically from the 22 cases out of 27,000 people that the FDA had reported. The researchers published their findings on the online newsletter platform Substack (see go.nature.com/3u97s38). “We wanted to get it out as soon as possible because of the timeliness of what was going on,” Fonseca says.
The race to supercharge cancer-fighting T cells
Since the FDA’s warning, Hall has started talking about the possibility of secondary cancers to individuals who are contemplating CAR-T therapy. He presents it as a real risk, but a rare one — and explains that it is dwarfed by the risk posed by their current cancer. “For my late-stage myeloma patient, the main risk is that the CAR T doesn’t work and they die of their myeloma,” he says. Mackall and others agree. “I don’t think anyone believes that this will change practice in any way at the current time,” she adds. “Most cancer therapies can cause cancer. This is one of the paradoxes of our business.”
But what about other diseases? Researchers have already tested CAR T cells as a therapy for the autoimmune condition lupus, with impressive results5. And more clinical trials of these therapies for other autoimmune diseases are likely to follow. If most of the secondary cancers seen in people treated with CAR T cells are related to the litany of treatments they received beforehand, people with these conditions might not all have the same risk. But even if the therapy is driving some cancers, many say the benefits might still be worth the risk. “Autoimmune diseases are not benign diseases,” said Marks in response to an audience question at an industry briefing in January (see go.nature.com/3jpk6qj). “Anyone who’s ever known somebody who’s had lupus cerebritis or lupus nephritis will know that those are potentially lethal diseases.”
CAR T cells also hold promise as a treatment for HIV infection, and a trial to test this idea kicked off in 2022. Researchers are also studying how CAR T cells could be used as a way to curb rejection of transplanted kidneys, or to clear out zombie-like senescent cells that have been implicated in ageing. The possibilities are continuously expanding.
As for whether the benefit of CAR-T therapy outweighs the risk of secondary cancers for these other indications, only time will tell.
[ad_2]
Source Article Link