[ad_1]
Hello Nature readers, would you like to get this Briefing in your inbox free every day? Sign up here.
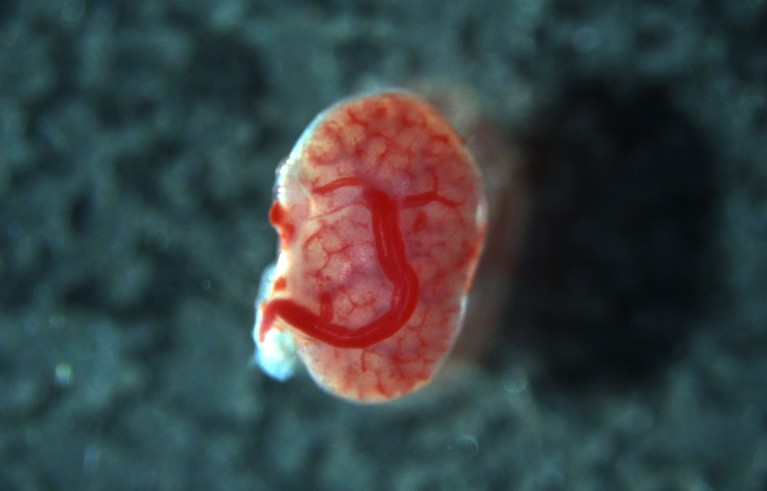
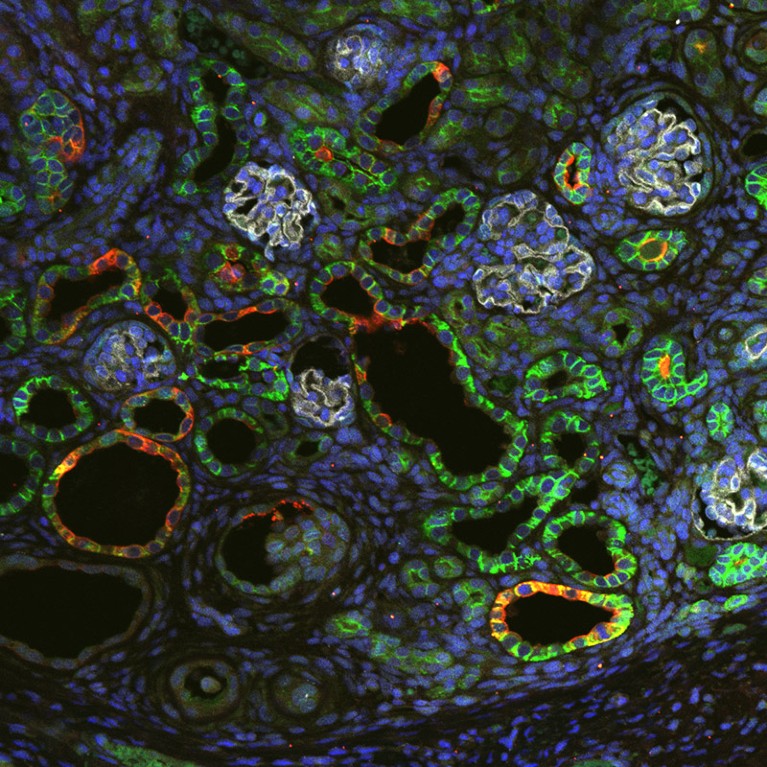
The transplanted tissue grew into a kidney with identifiable parts, and the host’s blood vessels began infiltrating it.Credit: K. Morimoto et al./bioRxiv
Surgeons have transplanted kidney tissue from one rat fetus to another, while the recipient was still in its mother’s womb. The recipient pups were born as normal after 22 days, according to a preprint (not peer reviewed). The transplanted tissue grew into an organ with some functioning characteristics of a kidney. Blood vessels from the host fetus grew inside the donated tissue, lowering the risk of rejection. Nephrologist and study lead Takashi Yokoo has also tested transplanting mouse kidney tissue into rat fetuses, with some success. His long-term goal, he says, is to one day transplant pig organs into human fetuses that have fatal kidney-development disorders.
Reference: bioRxiv preprint (not peer reviewed)
China is launching an ambitious mission later this week to collect rocks from the side of the Moon that we never see from Earth. Spacecraft Chang’e-6 was originally built as a backup for the Chang’e-5 mission, which successfully returned 1.73 kilograms of samples from the Moon’s near side in 2020. If Chang’e-6 pulls off a difficult soft landing, the lander will drill and scoop up two kilograms of soil and rocks from the more rugged far side. The samples might help explain why the two sides of the Moon are so different, including their history of volcanic activity. “When those samples come back to Earth, they will be like a Christmas present — whoever opens it will be happily surprised,” says planetary geologist Carolyn van der Bogert.
A free browser plug-in from source-checking company RedacTek flags when a paper cites studies that are mentioned on PubPeer, a forum often used to discuss issues such as image manipulation or plagiarism. PubPeer’s own browser plug-in also alerts users when a study has been posted on the site, but doesn’t search through the references. The new tool also highlights when a study or any paper that it cites has been retracted, and also scores papers according to the number of self-citations — references to authors’ own studies.
Reference: RedacTek Chrome browser plug-in
Researchers used a generative AI tool trained on millions of protein sequences to design CRISPR gene-editing proteins, and were then able to show that some of these systems work as expected in the laboratory. Another team developed a model trained on microbial genomes, and used it to design fresh CRISPR systems, which comprise a DNA or RNA-cutting enzyme and RNA molecules that tell the molecular scissors where to cut. Natural CRISPRs — part of some microbes’ immune system — have limitations on the genes they can edit and the changes they can make. “Expanding the repertoire of editors, using AI, could help,” says synthetic biologist Alan Wong.
Reference: bioRxiv preprint 1 & preprint 2 (not peer reviewed)
Features & opinion
Wildlife managers in Alaska are planning to parachute elite fire-fighters into remote areas to fight fires that threaten permafrost. In the Yukon Flats National Wildlife Refuge, an area the size of Denmark, fires have long been allowed to burn themselves out unless they threaten human life and property. But as climate change increases the frequency of blazes, the fear is that frozen permafrost will release its carbon as it thaws. A preprint (not peer reviewed) suggests that the resulting emissions could equate with those from a major global economy over this century. “What we’re talking about is aggressive attacks on fires when they ignite in these areas,” says Earth-systems scientist Brendan Rogers. Once such fires get going, it’s often too late: “That carbon is lost.”
Reference: Research Square preprint (not peer reviewed)
Chan Zuckerberg CELL by GENE Discover is a collection of free and open-source tools for finding, querying, analysing, downloading and publishing data from 85 million single cells. There are thousands of single-cell data sets scattered across the internet, but collecting and curating them can be “a huge pain in the butt”, says bioinformatician Timothy Triche Jr. “People underestimate the degree to which the impact of this data is amplified by making it usable for anybody who wants to.”
Edward Dwight, age 90, is expected to join a six-person crew aboard Blue Origin, on the private space company’s seventh mission beyond Earth’s orbit next month. Dwight first aimed for the stars 60 years ago, as a young US Air Force captain. He was selected by US President John Kennedy in 1961 to be the nation’s first Black astronaut, but was passed over by Chuck Yeager, the head of the test pilot programme. Dwight recalls Yeager as a racist who wanted him off the programme. He says he considers anger a waste of time, but counters those who see his upcoming flight as justice being served. “It seems far too late for it to be justice,” says Dwight. “This is a natural occurrence that should have happened at some point.”
The New York Times | 6 min read
Infographic of the week
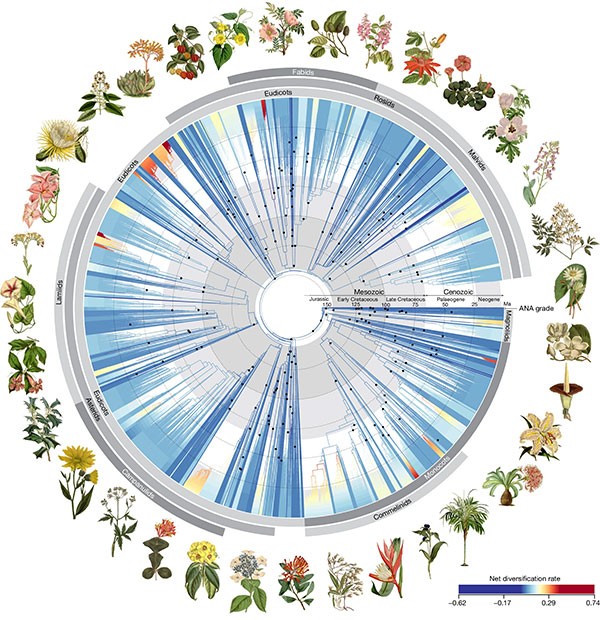
The tree of life for angiosperms — flowering and fruiting plants that make up 90% of plants on land — has been redrawn using the genomic data from more than 9,500 species. “Our very existences are dependent on them,” says systematic biologist and co-author William Baker. “That’s why we really need to understand them.” (New Scientist | 3 min read)
See a full-size version of this image here. (Alexandre R. Zuntini et al./Nature)
Today I’m discovering that song lyrics are getting simpler and more repetitive, according to an analysis of around 350,000 English-language pop, rock, rap, R&B and country songs released between 1970 and 2020. Further research is needed to determine who put the bomp in the bomp bah bomp bah bomp and who put the ram in the rama lama ding dong.
Share your favourite song lyric of all time with me, plus any feedback on this newsletter, at [email protected].
Thanks for reading,
Flora Graham, senior editor, Nature Briefing
With contributions by Katrina Krämer and Sarah Tomlin
Want more? Sign up to our other free Nature Briefing newsletters:
• Nature Briefing: Microbiology — the most abundant living entities on our planet — microorganisms — and the role they play in health, the environment and food systems.
• Nature Briefing: Anthropocene — climate change, biodiversity, sustainability and geoengineering
• Nature Briefing: AI & Robotics — 100% written by humans, of course
• Nature Briefing: Cancer — a weekly newsletter written with cancer researchers in mind
• Nature Briefing: Translational Research — covers biotechnology, drug discovery and pharma
[ad_2]
Source Article Link