[ad_1]
When Bente Klarlund Pedersen wakes up in the morning, the first thing she does is pull on her trainers and go for a 5-kilometre run — and it’s not just about staying fit. “It’s when I think and solve problems without knowing it,” says Klarlund Pedersen, who specializes in internal medicine and infectious diseases at the University of Copenhagen. “It’s very important for my well-being.”
Whether it’s running or lifting weights, it’s no secret that exercise is good for your health. Research has found that briskly walking for 450 minutes each week is associated with living around 4.5 years longer than doing no leisure-time exercise1, and that engaging in regular physical activity can fortify the immune system and stave off chronic diseases, such as cancer, cardiovascular disease and type 2 diabetes. But, says Dafna Bar-Sagi, a cell biologist at New York University, the burning question is how does exercise deliver its health-boosting effects?
“We know that it is good, but there is still a huge gap in understanding what it is doing to cells,” says Bar-Sagi, who walks on a treadmill for 30 minutes, five days a week.
In the past decade, researchers have started to build a picture of the vast maze of cellular and molecular processes that are triggered throughout the body during — and even after — a workout. Some of these processes dial down inflammation, whereas others ramp up cellular repair and maintenance. Exercise also prompts cells to release signalling molecules that carry a frenzy of messages between organs and tissues: from muscle cells to the immune and cardiovascular systems, or from the liver to the brain.
But researchers are just beginning to work out the meaning of this cacophony of crosstalk, says Atul Shahaji Deshmukh, a molecular biologist at the University of Copenhagen. “Any single molecule doesn’t work alone in the system,” says Deshmukh, who enjoys mountain biking during the summer. “It’s an entire network that functions together.”
Endurance exercise causes a multi-organ full-body molecular reaction
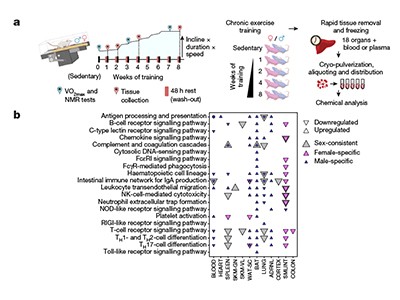
Exercise is also attracting attention from funders. The US National Institutes of Health (NIH), for instance, has invested US$170 million into a six-year study of people and rats that aims to create a comprehensive map of the molecules behind the effects of exercise, and how they change during and after a workout. The consortium behind the study has already published its first tranche of data from studies in rats, which explores how exercise induces changes across organs, tissues and gene expression, and how those changes differ between sexes2–4.
Building a sharper view of the molecular world of exercise could reveal therapeutic targets for drugs that mimic its effects — potentially offering the benefits of exercise in a pill. However, whether such drugs can simulate all the advantages of the real thing is controversial.
The work could also offer clues about which types of physical activity can benefit people with chronic illnesses, says Klarlund Pedersen. “We think you can prescribe exercise as you can prescribe a medicine,” she says.
Hard-wired for exercise
Exercise is a fundamental thread in the human evolutionary story. Although other primates evolved as fairly sedentary species, humans switched to a hunter-gatherer lifestyle that demanded walking long distances, carrying heavy loads of food and occasionally running from threats.
Those with better athletic prowess were better equipped to live longer lives, which made exercise a core part of human physiology, says Daniel Lieberman, a palaeoanthropologist at Harvard University in Cambridge, Massachusetts. The switch to a more active lifestyle led to changes in the human body: exercise burns up energy that would otherwise be stored as fat, which, in excess amounts, increases the risk of cardiovascular disease, type 2 diabetes and some cancers. The stress induced by running or pumping iron has the potential to damage cells, but it also kick-starts a cascade of cellular processes that work to reverse those effects. This can leave the body in better shape than it would be without exercise, says Lieberman.
Researchers have been exploring some of the biological changes that occur during exercise for more than a century. In 1910, pharmacologist Fred Ransom at the University of Cambridge, UK, discovered that skeletal muscle cells secrete lactic acid, which is created when the body breaks down glucose and turns it into fuel5. And in 1961, researchers speculated that skeletal muscle releases a substance that helps to regulate glucose during exercise6.
More clues were in store. In 1999, Klarlund Pedersen and her colleagues collected blood samples from runners before and after they took part in a marathon and found that several cytokines — a type of immune molecule — spiked immediately after exercise and that many remained elevated for up to 4 hours afterwards7. Among these cytokines were interleukin-6 (IL-6), a multifaceted protein that is a key player in the body’s defence response. The following year, Klarlund Pedersen and her colleagues discovered8 that IL-6 is secreted by contracting muscles during exercise, making it an ‘exerkine’ — the umbrella term for compounds produced in response to exercise.

Exercising regularly can strengthen the immune system and stave off disease.Credit: Mike Kemp/Getty
High levels of IL-6 can be beneficial or harmful, depending on how it is provoked. At rest, too much IL-6 has an inflammatory effect and is linked to obesity and insulin resistance, a hallmark of type 2 diabetes, says Klarlund Pedersen. But when exercising, the molecule activates its more calming family members, such as IL-10 and IL-1ra, which tone down inflammation and its harmful effects. “With each bout of exercise, you provoke an anti-inflammatory response,” says Klarlund Pedersen. Although some physical activity is better than none, high-intensity, long-duration exercise that engages large muscles — such as running or cycling — will crank up IL-6 production, adds Klarlund Pedersen.
Exercise is a balancing act in other ways, too. Physical activity produces cellular stress, and certain molecules counterbalance this damaging effect. When mitochondria — the powerhouses that supply energy in cells — ramp up production during exercise, they also produce more by-products called reactive oxygen species (ROS), which, in excessive amounts, can damage proteins, lipids and DNA. But these ROS also kick-start a horde of protective processes during exercise, offsetting their more toxic effects and fortifying cellular defences.
Among the molecular stars in this maintenance and repair arsenal are the proteins PGC-1α, which regulates important skeletal muscle genes, and NRF2, which activates genes that encode protective antioxidant enzymes. During exercise, the body has learnt to benefit from a fundamentally stressful process. “If stress doesn’t kill you it makes you stronger,” says Ye Tian, a geneticist at the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing.
Exerkines everywhere
Since IL-6 ushered in the exerkine era, the explosion of multiomics — an approach that combines various biological data sets, such as the proteome and metabolome — has allowed researchers to go beyond chasing single molecules. They can now begin untangling the convoluted molecular web that lies behind exercise, and how it interacts with different systems across the body, says Michael Snyder, a geneticist at Stanford University in California, who recently switched from running to weightlifting. “We need to understand how these all work together, because [humans] are a homeostatic machine that needs to be properly tuned,” he says.
In 2020, Snyder and his colleagues took blood samples from 36 people aged between 40 and 75 years old before, during and at various time intervals after the volunteers ran on a treadmill. The team used multiomic profiling to measure more than 17,000 molecules, more than half of which showed significant changes after exercise9. They also found that exercise triggered an elaborate ‘choreography’ of biological processes such as energy metabolism, oxidative stress and inflammation. Creating a catalogue of exercise molecules is an important first step in understanding their effects on the body, says Snyder.
How an exercise habit paves the way for injured muscles to heal
Other studies have probed how exercise affects cell types. A 2022 study in mice led by Jonathan Long, a pathologist at Stanford University, identified more than 200 types of protein that were expressed differently by 21 cell types in response to exercise10. The researchers were expecting to find that cells in the liver, muscle and bone would be most sensitive to exercise, but to their surprise, they found that a much more widespread type of cell, one that appears in many tissues and organs, showed the biggest changes in the proteins that it cranked out or turned down. The findings suggest that more cell types shift gears during a workout than was previously thought, although what these changes mean for the body is still an open question, says Long.
The findings also showed that after exercise, the mice’s liver cells squeezed out several types of carboxylesterase enzyme, which are known to ramp up metabolism. When Long and his colleagues genetically tweaked mice so that their livers expressed elevated levels of these metabolism-enhancing enzymes, and then fed them a diet of fatty foods, the mice didn’t gain weight. They also had increased endurance when they ran on a treadmill. “The improvement in exercise performance by these secreted carboxylesterases was not known before,” says Long, whose weekly exercise regime involves swimming and lifting weights. He adds that if the enzymes could be produced in the right quantities and purity, they could possibly be used as exercise-mimicking compounds.
During a workout, distant organs and tissues communicate with each other through molecular signals. Along with exerkines, extracellular vesicles (EVs) — nanosized, bubble-shaped structures that carry biological material — could be one of the mechanisms behind organ and tissue crosstalk, says Mark Febbraio, a former triathlete who is now an exercise physiologist at Monash University in Melbourne, Australia. In 2018, Febbraio and his team inserted tubes into the femoral arteries of 11 healthy men and drew blood before and after they rode an exercise bike at an increasing pace for an hour. During and after exercise, but not at rest, they found a spike in the levels of more than 300 types of protein that compose or are carried by EVs11.
When the team then collected EVs from mice that had run on a treadmill and injected them into another group of healthy mice, most of the EVs ended up in liver cells. In a separate mouse study that is yet to be published, Febbraio and his colleagues found hints that the contents of these liver-bound EVs can arrest a type of liver disease. A big question is whether EVs also deposit genetic material into different cells, and if so, what that means for the body. “We still don’t know a great deal,” he says.
Exercise as medicine
Larger efforts are under way to build a detailed molecular snapshot of how exercise exerts its health-boosting effects across tissues and organs. In 2016, the NIH established the Molecular Transducers of Physical Activity Consortium (MoTrPAC), a six-year study on around 2,600 people and more than 800 rats that aims to generate a molecular map of exercise. The effort — one of the largest studies on physical activity — is teasing apart the effects of aerobic and endurance exercise on multiple tissue types across different ages and fitness levels.
The first data set is from rats that completed one to eight weeks of treadmill training, and had blood and tissue samples collected at the end. The researchers pinpointed thousands of molecular changes throughout the rats’ bodies, many of which could have a protective effect on health, such as dialling down inflammatory bowel disease and tissue injury2. A separate study3 found that the effects of endurance training differed across sexes: markers associated with the breakdown of fat increased in male fat tissue, driving fat loss, whereas female fat tissue showed an increase in markers related to fat-cell maintenance and insulin signalling, which might protect against cardiometabolic diseases. A third study4 found that exercise alters the expression of genes linked to diseases such as asthma, and could help to trigger similar adaptive responses.
Focus on exercise metabolism and health
A big goal is to uncover why exercise has such varied effects on people of different sexes, ages and ethnic backgrounds, says Snyder, who is a member of the MoTrPAC team. “It’s very obvious that some people benefit better than others,” he says.
Researchers hope that the reams of molecular data will eventually help clinicians to develop tailored exercise prescriptions for people with chronic diseases, says MoTrPAC team member Bret Goodpaster, an exercise physiologist at the University of Pittsburgh in Pennsylvania. Farther down the track, such insights could be used to develop therapeutics that mimic some of the beneficial effects of exercise in people who are too ill to work out, he says. “That’s not to say that we will have exercise in a pill, but there are certain aspects of exercise that could be druggable,” says Goodpaster, who has taken part in triathlons, marathons and cycling races.
Several teams are already in the early stages of developing exercise-mimicking therapeutics. In March 2023, a team led by Thomas Burris, a pharmacologist at the University of Florida in Gainesville, identified a compound that targets proteins called oestrogen-related receptors, which are known to trigger key metabolic pathways in energy-intensive tissues, such as heart and skeletal muscle, particularly during exercise12. When the researchers administered the compound — called SLU-PP-332 — to mice, they found that the treated rodents were able to run 70% longer and 45% farther than untreated mice. Six months later, a separate study, also led by Burris, found that obese mice treated with the drug lost weight and gained less fat than those that didn’t receive the treatment — even though their diet was the same and they didn’t exercise any more than usual13.
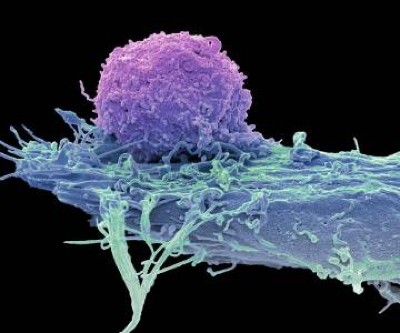
There is already evidence that exercise itself acts like medicine. In 2022, Bar-Sagi and her colleagues found that mice with pancreatic cancer had elevated levels of CD8 T cells — which destroy cancerous and virus-infected cells — when they did 30 minutes of aerobic exercise for 5 days a week14. These killer cells express a receptor for IL-15, another exerkine released by muscles during exercise. The researchers found that when CD8 T cells bind to IL-15, they unleash a more powerful immune response on tumours in the pancreas. This effect prolonged survival of mice with tumours by around 40%, compared with that of control mice. The findings held up when Bar-Sagi and her team analysed tumour tissue taken from people with pancreatic cancer. Those who did 60 minutes of aerobic and strength training each week had more CD8 T cells, and were twice as likely to survive for up to 5 years, than were people in the control group.
Although exercising more is a no-brainer for improving health, around 25% of adults globally do not meet the World Health Organization’s recommended levels of exercise each week: 150–300 minutes or more of moderate-intensity exercise, such as a brisk walk; or 75–150 minutes of vigorous-intensity exercise, such as running. David James, an exercise physiologist at the University of Sydney in Australia, who rides his bike to work each day, says that understanding the inner workings of exercise could help to develop clearer public-health messages about why physical activity is important and how it can offset the risk of getting chronic diseases. “That’s a powerful message,” says James.
[ad_2]
Source Article Link