[ad_1]
Female animals and women have been ignored or actively excluded in clinical and laboratory-based biomedical research since such research began. This was especially true until the US Congress passed the National Institutes of Health (NIH) Revitalization Act in 1993, which directed the NIH to establish guidelines on the inclusion of women and members of under-represented racial and ethnic groups in clinical trials.
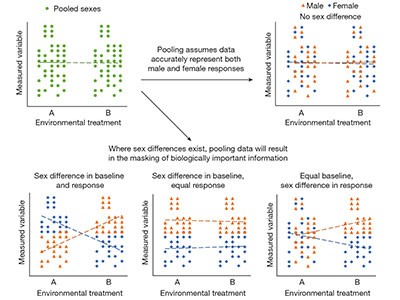
By 2009, a review of 10 fields in biology found that more than 60% of studies with human participants reported on both sexes. For studies using non-human animals, however, only 26% included both male and female subjects1.
To try to correct this persistent imbalance, the NIH implemented extra guidelines in 2016 — this time, on the inclusion of sex as a biological variable in all preclinical research2. At least with respect to the inclusion of female individuals in basic research, this funding-agency mandate and others like it have been effective. Another bibliometric analysis found that 49% of 720 studies on animals published in 2019 used both males and females3.
Why it’s essential to study sex and gender, even as tensions rise
Although it is still early days and there is much room for improvement, the inclusion of female participants and animal subjects is already having a revolutionary impact on numerous areas of study — from chronic pain to mental health. Yet we see an impending collision between research policies and societal changes regarding ideas and attitudes around sex and gender that threatens this nascent enterprise. We also see the threat of lobbyists, legislators and others in the United States and elsewhere weaponizing research on sex differences — either to marginalize individuals or groups that they deem to be outside a narrowly defined norm, or to reinforce derogatory ideas about people who identify as divergent4. (In this article, sex refers to differences between females and males caused by biological factors, whereas gender refers to differences caused by social factors, including gender roles, expectations and identity.)
Our concern is that various critiques of research on sex differences from scholars approaching sex and gender from different viewpoints — in combination with valid concerns around the misinterpretation or misuse of findings — could undermine an approach that has proved both practical and powerful. As a counterweight to this possibility, here we argue for the ongoing value of comparing female and male individuals in biomedical research.
Mammalian biology
Several scholars have argued in recent years that an overemphasis on biological sex will distract investigators from the effects of gendered environments and of non-sex-related variables, such as age, ethnicity or socio-economic status, on many traits. Another common criticism is that comparing female and male participants ignores transgender people and other individuals who do not fall within these binary categories, leading to their further marginalization in society5. Others have argued that a focus on the difference between the mean values of male and female individuals distracts researchers from considering the variability around those means — the implication being that variability within a sex is more important than variability between sexes. Some even question whether sex is a viable concept6.
Before addressing these specific complaints, it is worth briefly reviewing the current understanding of mammalian biology as it relates to sex — as well as some of the diverse and surprising findings that have already emerged from research comparing two sexes.
We need more-nuanced approaches to exploring sex and gender in research
Sex has been with us since our species originated as a result of sexual reproduction. The division of humans and other mammals into two sexes, female and male, derives from the fact that each individual is created by the union of a sperm and an egg. On the basis of the type of germ cell (gamete) that reproducing individuals are able to produce, there are only two sex categories in mammals. (Intersex is not a third category with respect to the type of gamete individuals can produce.) Indeed, understanding of how the mammalian genome evolved and how it functions is based on the foundation of sexual reproduction.
In mammals, as in many other taxa, the biological difference between sexes starts with the genetic difference encoded by the sex chromosomes — typically XX and XY in mammals — which are the only features that differ in female and male zygotes at the beginning of life. The salient role of the sex chromosomes is determining whether the embryo will develop ovaries or testes, because this specifies the type of germ cell that will be made, and the level and secretory patterns of testicular or ovarian hormones. Sex-chromosome genes and gonadal hormones influence almost every tissue in the body. The result might be sex differences in tissue development and function, or similar phenotypes based on different underlying mechanisms7.

Sex differences in immune function might have arisen from the need for female organisms to transfer immunity to the next generation.Credit: Klein & Hubert/Nature Picture Library
As in all things in biology, in humans and other mammals there are variations in the number and type of sex chromosomes and in the downstream mechanisms determining the phenotypic features associated with sex. This leads to variability among individuals in diverse sex-related traits, such as genital anatomy, body size and some behaviours. Also, particularly in humans, biological factors that drive sex differences in cells and tissues are confounded by social and environmental factors that also cause differences between individuals.
To serve all individuals equitably — including those who experience an incongruency between the sex they were assigned at birth and their current gender identity, and those who do not find that they align with either the male or female sex category — the medical profession and biomedical community must identify and interrogate these variations in biological attributes and in lived experiences, all of which can influence people’s physiology, risk of developing disease and prognosis8. This includes carefully attending to the distinctions between cisgender, transgender and non-binary individuals when reporting findings.
Yet we maintain that, in humans and other mammals, the comparison of individuals who have XX chromosomes and ovaries with individuals who have XY chromosomes and testes is a necessary component of basic and clinical research that seeks to improve human health.
Rich pickings
Male and female individuals represent most of the mammalian population. And research regarding biological sex differences has focused first on the largest groups, but in a manner that provides insights about variation within and beyond the binary.
For example, investigators have manipulated factors such as gonadal hormones and sex-chromosome genes to test their effects on sexual differentiation and their role in sex differences in disease. These manipulations, which mimic numerous intersex variations, such as the presence of ovarian hormonal secretions in an individual with XY chromosomes, have shed light on the effects of hormones, sex-chromosome genes and other factors in everyone. Studies of people with a variety of naturally occurring hormonal and chromosomal differences, for instance, are consistent with the interpretation that prenatal exposure to androgens, such as testosterone, is an important component of male psychosocial development9.
Nature journals raise the bar on sex and gender reporting in research
Importantly, the study of female and male individuals, as defined here, establishes a baseline measurement against which to compare findings from those who do not fit into a binary categorization scheme.
Understanding the effects of sex also anchors discussions about how different gendered environments intersect with biological differences, to amplify or mitigate their effects. More than half a century of animal research has been key to developing concepts of mammalian sexual differentiation, because in animals, unlike in humans, researchers can manipulate single genes or molecules to observe their effects on phenotypes. Moreover, although numerous environmental or social effects can be manipulated and studied in animals, such as diet, stress and levels of interaction with other individuals, animals provide useful models of the biological effects of sex in the absence of hard-to-control human gendered variables, such as cultural norms and expectations around child care and work.
The power of comparing female and male individuals in biomedical research is demonstrated most convincingly, however, by the data themselves — as illustrated by four examples from our fields of expertise.
Sex chromosomes versus hormones. Until recently, all of the biological hypotheses proposed to explain the significant sex differences in body weight and metabolism found in humans and animals (including birds and other mammals) were centred on the action of hormones. And extensive research during the twentieth century supported the idea that, in mammals, almost all sex differences in tissues other than the gonads (the organs that produce the gametes) result from the effects of ovarian and testicular hormones.
By the early 2000s, researchers studying gonadal development had created mouse models in which the complement of sex chromosomes could be manipulated in individuals with the same type of gonad10. This meant that investigators could assess whether the sex chromosomes cause differences in phenotypes, even when levels of gonadal hormones are similar7. Studies using the modified mice, while confirming the importance of gonadal hormones in influencing body weight and metabolism, uncovered the effects of sex chromosomes11. Comparable studies have also shown that sex chromosomes have much broader effects on physiology and behaviour than was originally thought10.
Accounting for sex and gender makes for better science
The copy number of an X-linked gene called Kdm5c, for example, contributes to a sex difference found in mice in the metabolism of adipose cells12. Mice with XY chromosomes have one copy of Kdm5c. They also have less body fat than do mice with XX chromosomes, which have two copies of the Kdm5c gene.
Over the past two decades, investigators have found that similar sex-chromosome effects contribute to sex differences in many other physiological systems in mice. And these sex differences, in turn, affect individuals’ likelihood of developing autoimmune conditions, cardiovascular diseases, cancer and developmental defects in the neural tube, the embryonic precursor to the central nervous system. The X-linked gene Kdm6a, for instance, increases the severity of autoimmune disease, and protects against bladder cancer and an Alzheimer’s-like disease in XX mice7. Similarly, the Y-linked gene Uty protects against pulmonary hypertension in mice13. Sex-chromosome genes also affect mouse behaviour, from the social behaviour of juveniles to responses to pain, as well as the size of certain brain regions7,10.
All of this work in mice provides investigators with clues about where to look for potential therapeutic targets in the human genome, for diseases that tend to affect women and men differently.
Pain. It is well established that among people with chronic pain, women far outnumber men14. Also, in experimental settings, women tend to be more sensitive than men are to pain — induced, for instance, by the application of heat, cold or pressure.
Pain researchers have proposed various gender-based and sex-based explanations for these differences14, such as that women are more likely than men to go to the doctor, as shown by usage rates for health-care services. However, investigations in male and female mice have suggested that, at least in rodents, different mechanisms are responsible for the processing of persistent pain in females and males.
Why the sexes don’t feel pain the same way
A 2015 study in mice15, for example, and follow-up findings demonstrated that a well-studied mechanism for the processing of persistent pain — involving immune cells called microglia — operates only in male rodents. (It is well studied in males, at least.) In males, the microglia release a factor that causes neurons in the spinal cord to increase their firing, which sustains chronic pain. Although female mice have just as many microglia as male mice do, their microglia don’t seem to be involved in the pain circuit — or, if they are involved, it is in a more complicated way. In fact, in females, T cells might play a similar part to microglia in males.
Whether the microglial or T-cell mechanism for the processing of persistent pain is engaged in any one individual seems to be due to testosterone levels being above or below a certain threshold. This dimorphism suggests that different physiological mechanisms could contribute to some of the differences observed in men and women in relation to chronic pain.
Immune function. Numerous studies that involve comparing immune responses in female and male organisms — whether they are fruit flies, fish, lizards, birds or mammals — have shown that females often generate more robust immune responses to antigens than do their male counterparts16. This suggests that sex differences in immune function are evolutionarily conserved, perhaps because of a common need for female individuals to transfer immunity to the next generation (whether through breast milk or a yolk sac), or because of some other sex-specific selective pressure.
In humans, these immunological stimuli can be self-antigens (proteins made by our own cells), allergens, cancerous cells or pathogenic microbes. Because women have larger immune responses than men, they are more likely to develop autoimmune diseases and allergies, but less likely to be diagnosed with non-reproductive cancers, such as skin or colon cancer17, and certain infectious diseases, such as tuberculosis16 and COVID-1918.
Some studies suggest that women generate a greater immune response to certain vaccines than do men.Credit: Patrick Meinhardt/Bloomberg/Getty
The difference between female and male organisms in the amount of antibodies produced in response to immunological stimuli changes across the life course, being most robust during the reproductive years19. This could explain why females of reproductive age often generate more antibodies in response to vaccines and microbes than males do20, and why female antibody responses are more durable and cross-reactive against diverse variants, such as different strains of influenza virus.
Mouse models have shown that gonadal hormones contribute more to mammalian sex differences in vaccine-induced immunity than do genes linked to sex chromosomes, at least against influenza viruses21. In both mice and humans, concentrations of estradiol (a hormone that is typically produced at higher levels in female organisms) are positively associated with greater antibody responses to influenza vaccines22. In short, a wealth of insights about the benefits (and downsides) of a bolstered immune response have emerged only because researchers have compared immune responses in male and female organisms.
Mental health. Sex and gender differences in the prevalence of mental-health disorders in humans span the life course. Prepubescent boys are significantly more likely than prepubescent girls to be diagnosed with autism spectrum disorder or attention deficit and hyperactivity disorder23. In their late teens or early 20s, men are more likely to be diagnosed with early-life schizophrenia. They are also more likely to experience a brain injury caused by a lack of oxygen at birth, and to have neurological conditions, such as Tourette’s syndrome. After puberty, however, disorders involving depression, anxiety, compulsion and obsession are more frequent in women23.
Sociocultural factors probably contribute to the differences in the prevalence of many of these conditions, including biases around the criteria used to diagnose early-life disorders by clinicians. Similarly, by the time a woman is diagnosed with a mood or affective disorder, she has often lived for decades in a gendered environment, making it hard for researchers to separate the effects of biology during development from those of life experience. Studies conducted over the past two decades in male and female rodents, however, have revealed an integral role for the immune system — specifically microglial cells — in affecting how testosterone acts on the brain and alters the structure and function of certain regions.
The fraught quest to account for sex in biology research
For instance, experiments measuring cellular activity in post-mortem animals have shown that during development, male rodents have a greater number of activated microglia in certain regions of their brains than do female rodents. These activated microglia release more of the signalling molecules that are crucial to forming synapses and controlling cell numbers. Many of the brain regions affected by the selective elimination of cells are also those implicated in mental-health disorders in humans (in both sexes) that originate during development24.
These findings could offer clues as to why messenger RNAs obtained from the cortex of human male fetuses indicate higher expression levels of genes involved in inflammation than do those obtained from human female fetuses. Post-mortem, higher levels of inflammation have even been found in the cortices of men who had been diagnosed with autism than in those of men who had not received a mental-health diagnosis25.
All of this suggests that, in mammals, greater activity of the neuroimmune system is somehow involved in the process of brain masculinization — which means that various mental-health disorders that affect boys more than girls could involve disruptions to immune-system processes.
Early days
Ultimately, we support efforts to interrogate both biological and social determinants of disease. Indeed, having more information is always preferable to having less. It is crucial to consider how biological factors linked to sex interact with each other and with other biological factors, such as age and genetic background, as well as with sociocultural or environmental influences. But whether the variables that have the most impact on physiology and disease are sex-based, gender-based or unrelated to either is a question that must be answered by research.
Sex and gender analysis improves science and engineering
Related to this, although there is always a danger of scientists and journalists oversimplifying things — particularly in relation to sex and gender — any rigorous analysis requires the consideration of averages as well as measures of variation. Just as with the importance of sex-related variables compared to other variables, it is an empirical question whether within-sex variation has more or less impact on a trait of interest than between-sex variation does.
When it comes to the threat of people misusing statements about an inherent difference between female and male individuals to rationalize continuing the historical subordination of women, transgender people and others, we agree that this danger is real and urgent. Since September 2023, for instance, health-care providers in Texas have been prohibited from giving gender-transition surgeries, puberty-blocking medication or hormone therapies to people under 18. This was decided on the basis of claims that everyone belongs to one of two groups, and that this reality is settled by science. The solution, however, is not to deny a priori the importance of sex differences, but rather to improve understanding of variation in human populations and how it relates to biological and social factors. Similarly, whereas we recognize the importance of studying intersex, non-binary, transgender and other individuals whose biology or life experiences are not encompassed by a simplistic binary, the neglect of such individuals should not be addressed by abandoning female–male comparisons.
Because female organisms have for so long been left out of investigations in many biomedical fields, researchers are still surprisingly ignorant of their fundamental biology across numerous taxa, and how it does or does not differ from that of males. There is also much room for improvement in research on sex differences — in terms of statistical and reporting practices26, researchers actually splitting their data by sex and analysing those data appropriately3, and journals improving their policies around sex and gender. The highly fruitful approach of comparing female and male organisms should not be abandoned just as investigators are starting to make progress.
[ad_2]
Source Article Link