[ad_1]
Stem-cell researcher Carolina Florian didn’t trust what she was seeing. Her elderly laboratory mice were starting to look younger. They were more sprightly and their coats were sleeker. Yet all she had done was to briefly treat them — many weeks earlier — with a drug that corrected the organization of proteins inside a type of stem cell.
When technicians who were replicating her experiment in two other labs found the same thing, she started to feel more confident that the treatment was somehow rejuvenating the animals. In two papers, in 2020 and 2022, her team described how the approach extends the lifespan of mice and keeps them fit into old age1,2.
The target of Florian’s elixir is the immune system. The stem cells she treated are called haematopoietic, or blood, stem cells (HS cells), which give rise to all immune cells. As blood circulates, the mix of cells pervades every organ, affecting all bodily functions.
But the molecular composition of the HS cells changes with age, and this distorts the balance of immune cells that they produce. “Fixing the drift in them that occurs with time seems to fix a lot of the problems of ageing — not only in the immune system but also in the rest of the body,” says Florian, who is now at the Bellvitge Biomedical Research Institute in Barcelona, Spain.
In March3, another team showed that restoring the balance between two key types of immune cell gives old mice more youthful immune systems, improving the animals’ ability to respond to vaccines and to stave off viral infections.
How to make an old immune system young again
Other scientists have used different experimental approaches to draw the same conclusion: rejuvenating the immune system rejuvenates many organs in an animal’s body, at least in mice. And, most intriguingly, evidence suggests that immune-system ageing might actually drive the ageing of those organs.
The potential — helping people to remain healthy in their later years — is seductive. But translating this knowledge into the clinic will be challenging. Interfering with the highly complex immune system can be perilous, researchers warn. So, at first, pioneers are setting their sights on important yet low-risk goals such as improving older people’s responses to vaccinations and improving the efficiency of cancer immunotherapies.
“The prospect that reversing immune ageing may control age-related diseases is enticing,” says stem-cell scientist Vittorio Sebastiano at Stanford Medical School in California. “But we are moving forward cautiously.”
Fading immunity
The human immune system is a complex beast whose multitudinous cellular and molecular components work together to shape development, protect against infections, help wounds to heal and eliminate cells that threaten to become cancerous. But it becomes less effective as people age and the system’s composition starts to change. In older age, people become susceptible to a range of infectious and non-infectious diseases — and more resistant to the protective power of vaccines.
The immune system has two main components: a fast-acting innate system, which destroys invading pathogens indiscriminately, and a more-precise adaptive immune system, whose components learn to recognize specific foreign bacteria and viruses and generate antibodies against them.
The HS cells in the bone marrow spawn the immune cells of both arms of the system. They differentiate into two main classes — lymphoid and myeloid — which go on to differentiate further. Lymphoid cells are mostly responsible for adaptive immunity, and include: B cells, which produce antibodies; T cells, which help to attack invaders and orchestrate complex immune responses; and natural killer cells, which destroy infected cells. Myeloid cells include a raft of cell types involved mostly in innate immunity.
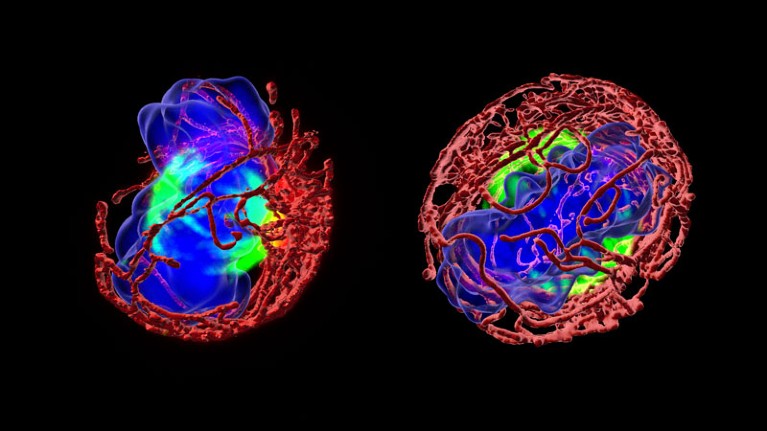
Proteins inside immune-cell-generating stem cells become more symmetrical with age (right).Credit: Eva Mejia-Ramirez
One of the earliest changes in the immune system as people age is the shrinking of the thymus, which begins after puberty. This organ is the crucible for T cells, but a lot of the tissue has turned to fat by the time people hit their 30s, slashing the production of new T cells and diminishing the power of the immune system. What’s more, the function of T cells alters as they age and become less specialized in their ability to recognize infectious agents.
The proportions of different types of immune cell circulating in the blood also changes. The ratio of myeloid to lymphoid cells skews markedly towards myeloid cells, which can drive inflammation. Moreover, increasing numbers of immune cells become senescent, meaning that they stop replicating but don’t die.
Any cell in the body can become senescent, typically when damaged by a mutation. Once in this state, cells start to secrete inflammatory signals, flagging themselves for destruction. This is an important anticancer and wound-healing mechanism that works well in youth. But when too much damage accumulates with ageing — and immune cells themselves also become senescent — the mechanism breaks down. Senescent immune cells, attracted by the inflammatory signals from senescent tissue, secrete their own inflammatory molecules. So not only do they fail to clean up properly, but they also add to the inflammation that damages surrounding healthy tissue. The phenomenon is known as ‘inflammaging’.
“It becomes a terrible positive feedback — a never-ending dance of destruction,” says immunologist Arne Akbar at University College London.
And evidence suggests that this feedback loop is kicked off by the immune system. In a series of experiments in mice4, Laura Niedernhofer at the University of Minnesota in Minneapolis has shown that immune-cell senescence actually drives senescence in other tissues. “These cells are extremely dangerous,” she says.
Her team used genetic methods to eliminate an important DNA-repair enzyme in the immune system of the mice. The animals remained healthy until adulthood but then, unable to correct accumulating mutations, various types of immune cell started to become senescent.
A few months later, increasing numbers of cells in organs such as the liver and kidney also fell into senescence, and the organs showed signs of damage. These effects were all reversed when the scientists gave the mice immune cells from the spleens of young, healthy mice.
All of this suggests that fixing the characteristics of immune-system ageing could help to prevent or mitigate diseases of ageing, says Niedernhofer.
Battling senescence
Many scientists are trying to do just that, from very different angles. Lots of the approaches hint that very short treatments of the immune system might have long-term effects, keeping side effects to a more manageable minimum.
One approach is to tackle senescent immune cells head on, using drugs to either remove them or block the inflammatory factors they secrete. “Senescent immune cells have long been known to be very modifiable in humans,” says Niedernhofer. “They go up if you smoke and down if you exercise.”
Are your organs ageing well? The blood holds clues
Some drugs — such as dasatinib, which is approved for the treatment of some cancers, and quercetin, which is marketed as an antioxidant dietary supplement but not approved as a drug — are known to reduce the age-related acceleration of senescence, and dozens of clinical trials are testing their impact on various age-related diseases. Niedernhofer herself is involved in a small clinical trial on older people with sepsis, a condition that becomes more deadly with age.
Her team is also doing experiments to assess which of the many types of immune cell is the most important in driving senescence in the body, which should help in the design of more precise therapies. Two types — T cells and natural killer cells — are emerging as key contenders, she says. She plans to screen natural products and drugs already approved for use by the US Food and Drug Administration for their ability to interact with those types of immune cell in senescence.
Akbar thinks that targeting inflammation itself might be as effective as targeting the senescent cells. He and his colleagues did a study in healthy volunteers using the investigational compound losmapimod, which blocks an enzyme involved in the production of inflammatory molecules called cytokines. They treated the volunteers with the drug for four days, and then, over the course of a week, measured their skin responses to an injection of the virus that causes chickenpox. Most people are exposed to this virus during their lives and it frequently lingers in the body. But with age, people tend to lose their immunity to it, and it can then manifest as shingles. The drug restored the immune response in the skin in older volunteers to a level similar to that seen in the younger volunteers5. In unpublished work, Akbar has found the same robust skin results up to three months later.
“Temporarily blocking inflammation in this way to allow the immune system to function might similarly boost the response of older patients to flu vaccinations,” says Akbar.
Immune boost
The value of priming the aged immune system before administering a vaccine has been demonstrated in a series of clinical trials led by researcher Joan Mannick, chief executive of Tornado Therapeutics, which is headquartered in Boston, Massachusetts. Those trials tested analogues of the drug rapamycin and other drugs with similar mechanisms, which target the immune system and are approved for prevention of organ transplant rejection and for the treatment of some cancers. The drugs block an enzyme, called mTOR, that is crucial for many physiological functions and which becomes dysregulated in old age.
For several weeks before receiving their influenza vaccinations, trial participants were treated with doses of the drugs that were low enough to avoid side effects. This treatment regimen improved their responses to the vaccine, and boosted the ability of their immune systems to resist viral infections in general.

Vaccines tend to work less efficiently in older adults, but new approaches could boost their power.Credit: Hector Vivas/Getty
But rapamycin can raise susceptibility to infection and affect metabolism, so Mannick is planning trials with similar drugs that might have a safer profile. “But there are all sorts of different ways to try to improve the immune system,” she notes.
One other way is to try to restore the function of the thymus to maintain the production of new T cells. Immunologist Jarrod Dudakov at the Fred Hutchinson Cancer Center in Seattle, Washington, is researching the basic biology of thymus cells to try to work out how they regenerate themselves after stressful assaults. “It’s all a bit early to see how this understanding will translate into the clinic,” he says. But he thinks that maintaining the ability of the thymus to generate a broad repertoire of T cells will be “foundational”.
Others are trying to combat ageing by generating thymic tissue from pluripotent stem cells for eventual transplantation. But Greg Fahy, chief scientific officer at Intervene Immune in Torrance, California, says he sees no need to wait for these long-term prospects to come to fruition, because an available drug — synthetic growth hormone — is already known to regenerate thymus tissue. He is doing a series of small studies on healthy volunteers using growth hormone as part of a cocktail of compounds. Early results indicate that the participants show increased levels of functional thymic tissue, and that their epigenetic clock — a biomarker of ageing — reverses by a couple of years6. Fahy is now extending the trial to look at whether the drug cocktail also improves physical fitness in a larger group of volunteers.
Turn back time
Another approach, not yet in the clinic, is to partially reprogram immune cells, to try to turn back the clock in cells that have become senescent. This involves transiently exposing the cells in a dish to a cocktail of transcription factors known to induce a pluripotent state in adult cells.
Reversal of biological clock restores vision in old mice
Sebastiano and his colleagues have shown in human cells that this process corrects the epigenetic changes that occur with ageing7. He has co-founded a start-up company to use the technique to try to counteract a problem in a cancer therapy known as CAR T, in which T cells are engineered outside the body to target and destroy a person’s cancer. But the T cells can turn senescent before they can be returned to the person. Rejuvenating them during the generation process would make production quicker and more robust, says Sebastiano.
Florian’s approach, too, aims to produce healthier immune cells — inside the body1,2. HS cells in the blood rack up epigenetic changes, and their environment also changes as they age. This causes proteins in the cells to arrange themselves more symmetrically — a process known as polarization — which shifts the balance of stem-cell differentiation in favour of myeloid cells over lymphoid cells. Florian’s studies used a four-day treatment with a compound, called CASIN, that inhibits one part of this process to correct the polarization, and helped the mice to live longer.
The team saw the same life-extending effects when HS cells from old mice given CASIN were transplanted into old mice that hadn’t received the treatment. “This very small step had a large impact,” says Florian.
Florian next hopes to bring her work to the clinic. As a first case study, she thinks her drug might support regeneration of the immune system after people receive chemotherapy for cancer.
How old?
Research on immune ageing faces some fundamental challenges. One is shared with ageing studies in all organs — the inability to measure ageing precisely.
“We don’t know in a quantitative, measurable, predictive way what ageing means at the molecular level in different cell types,” says Sebastiano. “Without those benchmarks, it is very hard to show rejuvenation.” Last year, a consortium of academics got together to begin developing a consensus on biomarkers of ageing — which will be essential when scientists come to seek approval from regulatory agencies for anti-ageing therapies.
Another challenge is the difficulty in pinning down what makes one immune cell unique. Until recently, it has been hard to demonstrate which subtypes of immune cells live where, and how they change with time.
But technologies such as single-cell RNA sequencing, which quantitatively measures the genes being expressed in individual cells, have tightened up analysis. A large study of immune cells in the blood of mice and humans across a range of ages published last November, for example, revealed 55 subpopulations. Just twelve of those changed with age8.
With so many strands of research coming together, scientists are cautiously hopeful that the immune system will indeed prove to be a key lever in healthy ageing. Don’t expect an elixir of youth any time soon, says Florian — by definition, ageing research takes a long time. “But there is such great potential for translation.”
[ad_2]
Source Article Link