[ad_1]

Credit: Taj Francis
In January 2023, the US Food and Drug Administration (FDA) approved lecanemab — an antibody medication that decreases β-amyloid protein build-up in the brain — as a treatment for Alzheimer’s disease. Pivotal evidence came from a large, randomized trial of people with early-stage Alzheimer’s, which afflicts around 32 million people worldwide. By the end of that 18-month study1, patients in the placebo group scored on average 1.66 points worse than their performance at baseline on a standard dementia test, which assesses cognitive and functional changes over time through interviews with a patient and their caregiver. The mean score of treated participants, by comparison, worsened by 1.21 points — a 27% slowing of cognitive decline.
But is this improvement meaningful for patients and their families?
Nature Index 2024 Health sciences
There are two major categories of drugs used to treat Alzheimer’s disease and other progressive conditions: symptomatic drugs, which treat the symptoms, and disease-modifying drugs, which target the root cause. Donepezil and rivastigmine, for example, are symptomatic drugs that boost the activity of chemicals in the brain to compensate for declines in cognitive and memory function caused by Alzheimer’s disease, but they cannot stop its progression. Lecanemab, developed jointly by Japanese pharmaceutical company Eisai and American biotechnology firm Biogen, targets the underlying issue of amyloid build-up in the brain, and in doing so, could fundamentally change the course of the disease.
An important feature of disease-modifying drugs is that their benefits are cumulative. Studies of patients with multiple sclerosis, for example, have shown the benefits of starting disease-modifying drugs earlier in the course of the disease compared with later, including improved mortality rates and reduced disability in the long term. Being able to quantify how long a disease-modifying drug can delay or halt the progression of Alzheimer’s disease could change how researchers understand — and communicate — its benefits.
In studies of potential disease-modifying drugs for Alzheimer’s disease, there has always been a tension between being able to produce a treatment effect and being able to measure it, says Suzanne Hendrix, statistician and founder of the clinical trials consulting firm Pentara in Salt Lake City, Utah. Clinical trials generally enrol early-stage patients — those with mild cognitive impairment and evidence of brain amyloid — because amyloid-targeting therapies have the best chance of working if given well before the disease takes hold. But in the early stages, patients deteriorate so gradually that it can be difficult to perceive the impact of a disease-modifying drug using standardized tests.
At a scientific meeting in 2009, Hendrix recalls being pulled aside by an executive at Eisai, who told her: “Nobody’s measuring this disease right. Until we measure the most progressive aspects of disease, we’re not going to be able to see treatment effects.”
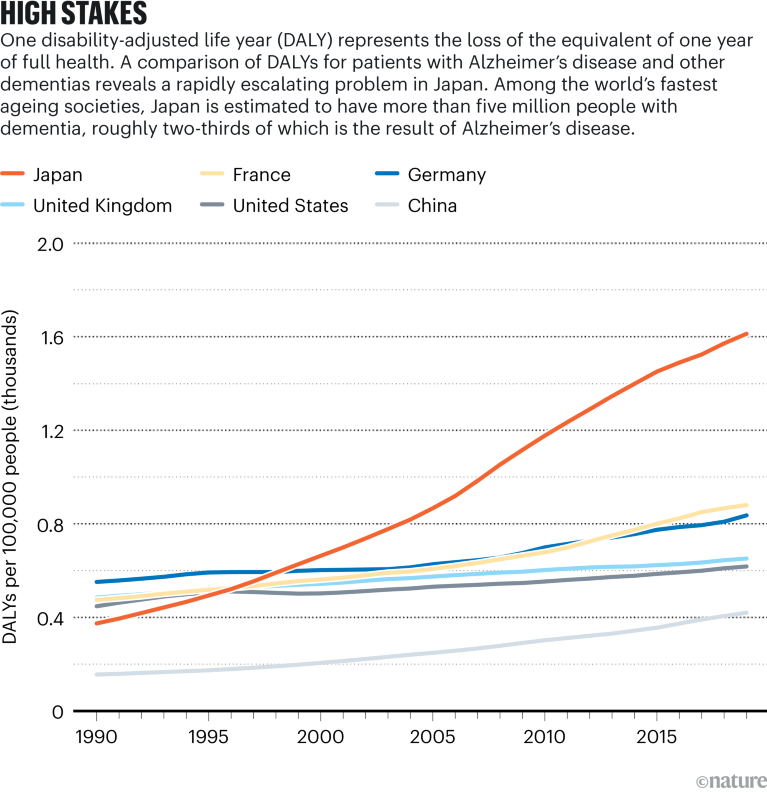
Source: Institute for Health Metrics and Evaluation; Cummings, J. L., Goldman, D. P., Simmons-Stern, N. R., Ponton, E. Alzheimers Dement. 18, 469–477 (2022)
Hendrix and other researchers are exploring time-based metrics as a new approach. Savings of time, measured as prolonged quality of life after 18 months of treatment, for example, is “much easier to talk about” than point differences on cognitive and functional scales, says Lars Rau Raket, a statistician at the Copenhagen, Denmark, branch of US pharmaceutical company Eli Lilly. For early-stage Alzheimer’s patients, says Racket, “it’s about how much you can extend the time in the ‘good parts’ — in the milder stages of disease”.
Straight line to time
To come up with a time-based approach, Hendrix and her colleagues pooled parts of several rating scales from standard dementia tests to develop a new tool called that picks up on subtle changes that occur in early Alzheimer’s. By zeroing in on where changes are more pronounced in these early stages, such as a diminished ability to juggle tasks or to recall past events, the team could track the progression of several key features of the disease.
To measure the effectiveness of disease-modifying treatments on these key features as units of time, the researchers used clinical outcomes from placebo and treated participants in a phase II trial of another amyloid-lowering therapy, donanemab. They calculated that over the 76-week duration of the trial, overall disease progression was delayed by 5.2 months.
In a paper published last year2, when he was working for Danish firm Novo Nordisk, in a lab just outside Copenhagen, Raket took a similar approach to calculating treatment effects in terms of time. But their methods differed in some ways. Whereas Hendrix’s work focused on calculating time savings across multiple outcomes, Raket used multiple models to calculate time savings for each outcome measure.
The idea of time-based models seems to be gaining traction. They were used as exploratory measures in a phase III trial of donanemab, conducted by Eli Lilly and Company, and published in JAMA last year3. Eisai also showed a time-based analysis in a 2022 presentation of its phase III lecanemab data at the Clinical Trials on Alzheimer’s Disease meeting in San Francisco. In those analyses, participants treated with lecanemab took 25.5 months to reach the same degree of worsening on a common dementia test as the placebo group did at 18 months — a time saving of 7.5 months.
Raket says he has been approached by several people in the pharmaceutical industry and academia, and some are working with him to apply the concept to their research. At the 2023 Alzheimer’s Association International Conference in Amsterdam, Raket and his collaborators in the United States, Canada and Europe compared time-based models with conventional statistical approaches for progressive diseases, and analysed how delays in disease progression calculated with time-based methods translate to treatment differences on standard cognitive tests. “I haven’t experienced this kind of interest in my work before,” he says. Raket predicts that an increasing number of trials in the neurodegeneration space will be reporting time-savings estimates in the years to come.
Broad impacts
Beyond Alzheimer’s disease, time-saved models could be applied to other progressive conditions, including Parkinson’s disease and amyotrophic lateral sclerosis (ALS). Cancer and cardiovascular disease studies, which tend to focus on events — delaying relapse or death, or cutting the risk of heart attacks, for instance — are less suited to models that track progression. If, however, heart disease were conceptualized as a gradual worsening of blood pressure or cholesterol over time, and treatment could be shown to slow the rate of deterioration, the time-saved approach could be used to measure the treatment benefit, says Hendrix.
One benefit of time-based methods is that they could help make clinical trials less prone to being skewed by outliers, says Geert Molenberghs, a biostatistician at KU Leuven and Hasselt University, both in Belgium, who collaborates with Hendrix. For example, a small subset of people with early Alzheimer’s disease deteriorate unusually quickly. If these rapid decliners are in the treated group, they could potentially mask a drug benefit, says Molenberghs. The details become “very technical”, he says, but with time-based approaches, these rare individuals “are less influential. They have less capacity to overturn the statistics.”
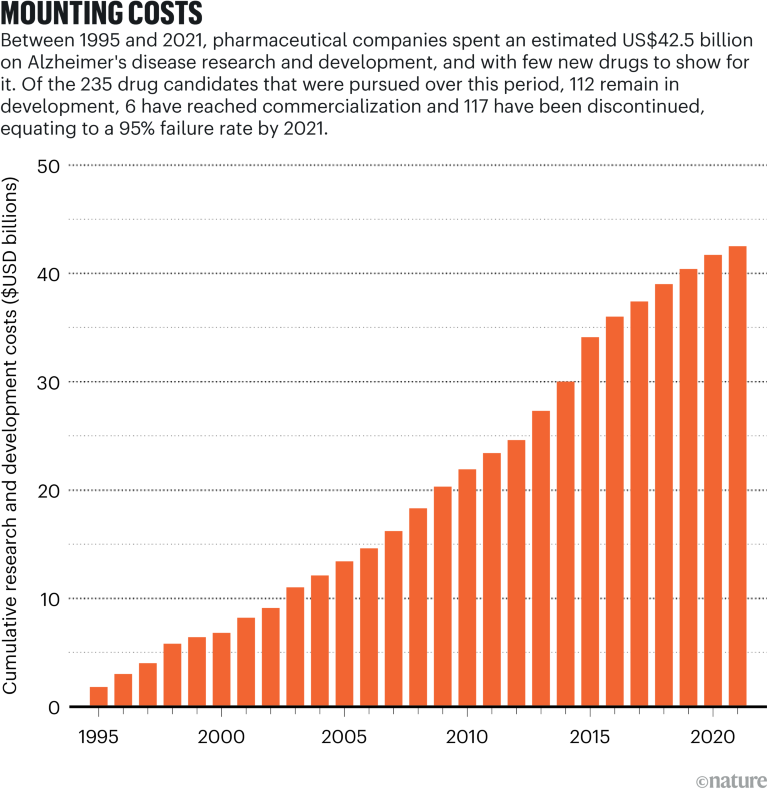
Source: Institute for Health Metrics and Evaluation; Cummings, J. L., Goldman, D. P., Simmons-Stern, N. R., Ponton, E. Alzheimers Dement. 18, 469–477 (2022)
Time-based metrics could impact broader conversations with health economists and policymakers. “The idea that you could take somebody who’s already in their senior years and keep them functional and not needing 24/7 care — that’s incredibly valuable information for making estimates about the true burden or cost of the disease to caregivers and society,” says John Harrison, chief science officer at Scottish Brain Sciences, a research institute in Edinburgh, Scotland. “It’s a very neat communications tool which feeds into estimates of progression, cost, strategy and, one hopes, legislation and planning.”
There are open questions that might need to be addressed before time-saved models are more widely applied in clinical trials. One is that, although time progresses linearly, not all points on that line are equally meaningful. For example, the anti-amyloid mechanism might only be beneficial in the early stages of Alzheimer’s disease, says Ron Petersen, a neurologist at Mayo Clinic in Rochester, Minnesota. “By the time the person progresses to, say, moderate dementia, modifying amyloid probably isn’t going to make any difference.”
Hendrix is hopeful that the time-saved idea can be further developed and applied to clinical trials in the future, because it could make a big difference in tracking not only how effective new disease-modifying drugs are, but also in helping Alzheimer’s patients and their families to better understand the progression of the disease and how they can plan for it.
Ultimately, as more studies “start focusing on how much time we’ve saved people, all of the effects that we see will be more relevant” to people’s daily lives, Hendrix says.
[ad_2]
Source Article Link


