[ad_1]
Vaccines are usually used to prevent infectious diseases. A therapeutic cancer vaccine is different. Rather than teaching the immune system to recognize pathogens in advance of an infection, these vaccines use identifying proteins produced by cancer cells, known as antigens, to provoke a powerful immune response to existing tumours.
A variety of approaches
The first step is to deliver antigens to immune cells called dendritic cells. These present antigens to other immune cells, and stimulate a response. In the past decade, several approaches have emerged1. One delivers antigens that are shared by many people with the same type of cancer (2). Others, including those that make use of messenger RNA (mRNA) technology, are highly personalized to the unique neoantigens produced by an individual’s tumour (3). Other personalized approaches involve injecting dendritic cells that are pre-loaded with cancer antigens (1), or generating antigens inside the body and promoting their uptake by dendritic cells in situ (4).
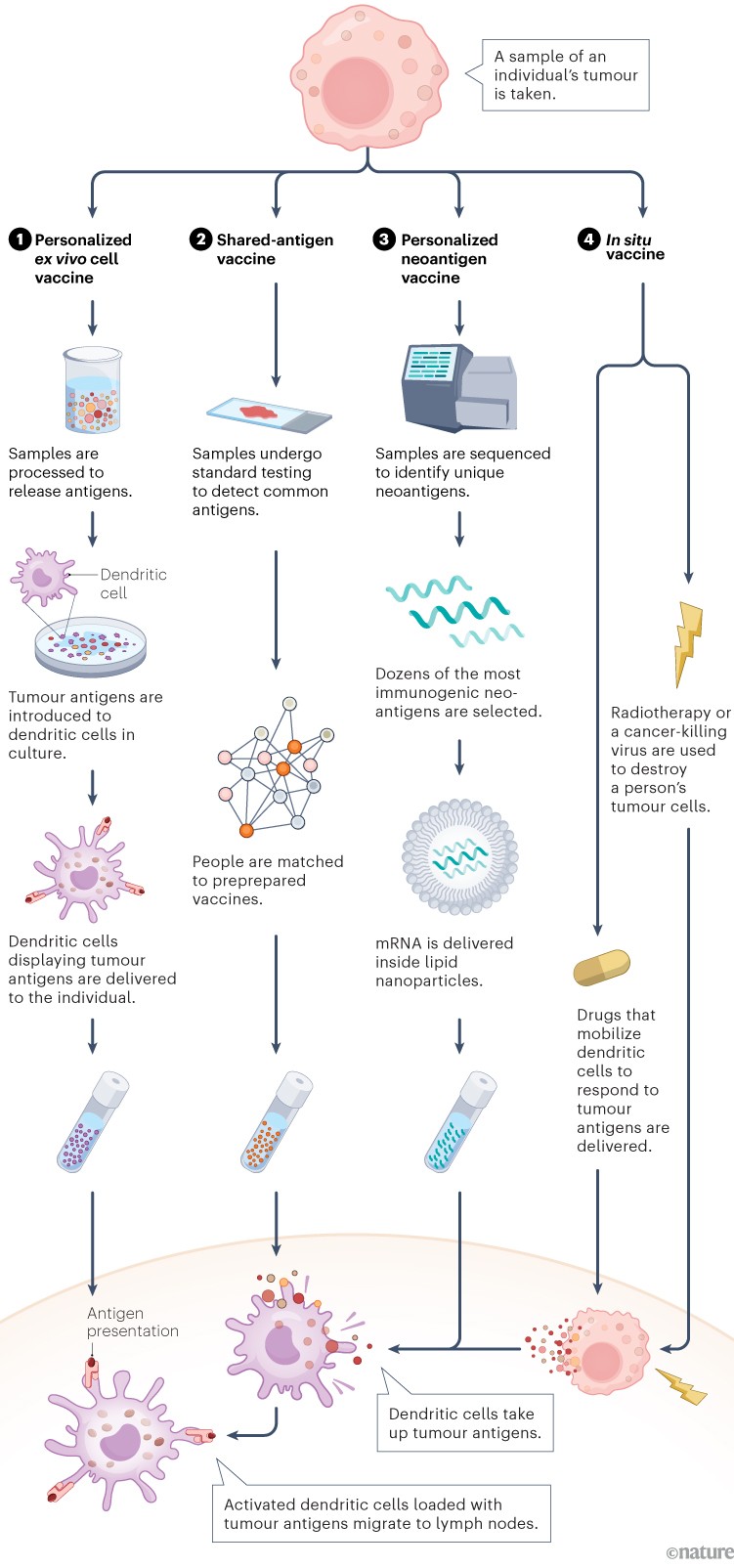
Infographic: Alisdair Macdonald
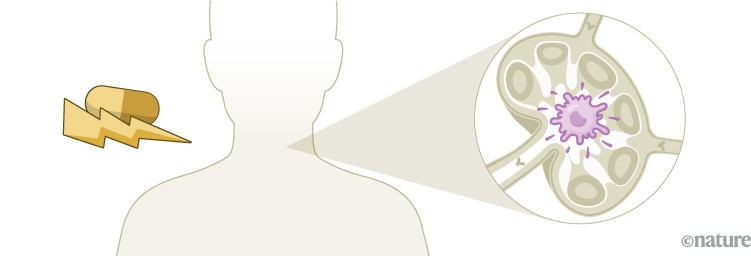
Mounting a response
Unlike preventive vaccines, which focus mainly on activating antibody-producing B cells, a therapeutic cancer vaccine must generate a strong T-cell response. Dendritic cells loaded with tumour antigens bind and activate CD8+ cytotoxic T cells, which can then mount an attack on the tumour2.
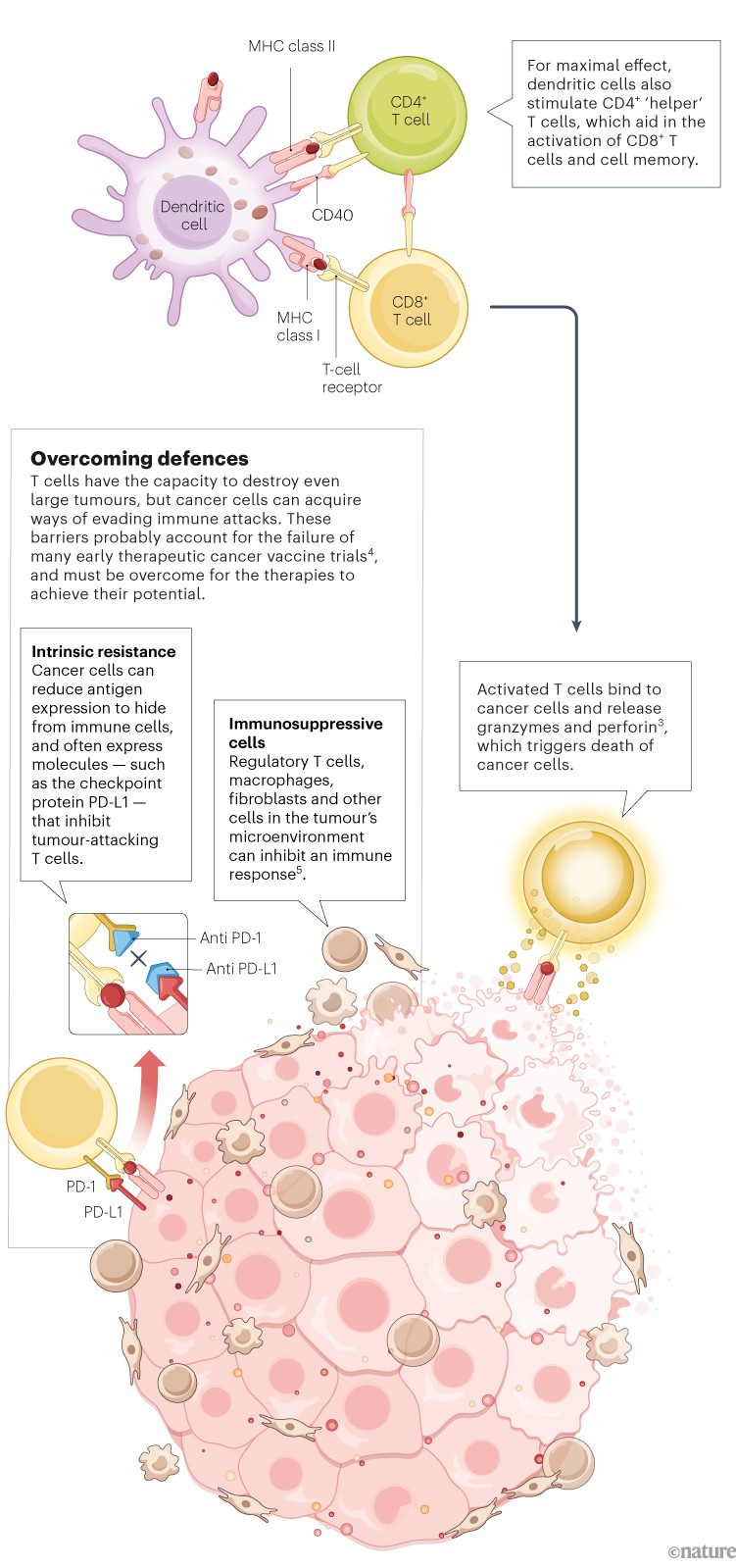
Infographic: Alisdair Macdonald
Promising results
Numerous therapeutic cancer vaccines, on the basis of a variety of approaches, are showing encouraging results in trials.
Pancreatic cancer: In a phase I trial of a personalized mRNA vaccine, half of the participants developed T cells targeted to cancer neoantigens6. Recurrence-free survival in this group was longer compared with those who did not respond.
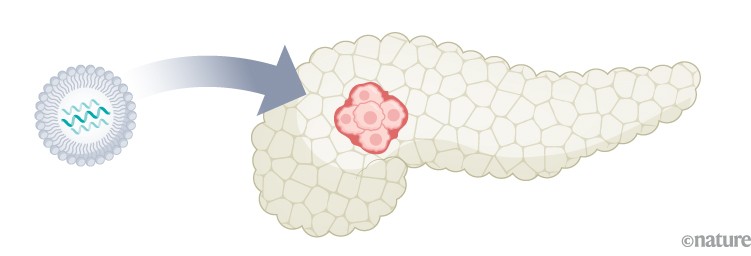
Infographic: Alisdair Macdonald
Melanoma: A phase II trial of a personalized mRNA vaccine showed a 44% decrease in the risk of post-surgical recurrence or death7. A phase III trial is under way, with final results expected in 2029.
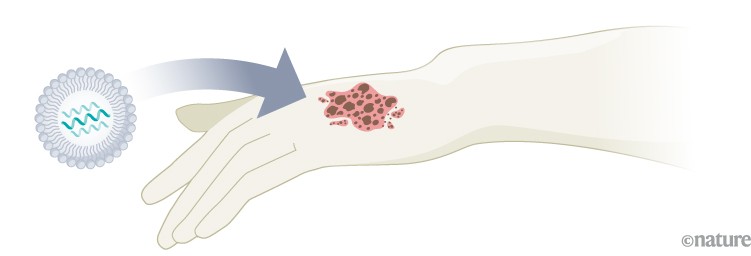
Infographic: Alisdair Macdonald
Lymphoma: A phase I/II trial of an in situ vaccine that combined radiotherapy with signalling molecules that mobilize and activate dendritic cells showed evidence of tumour regression in 8 of 11 people who were treated8.
Infographic: Alisdair Macdonald
Obstacles ahead
The future development and the clinical uptake of therapeutic cancer vaccines will be shaped by several factors.

Infographic: Alisdair Macdonald
Unwieldy trials. Testing multiple combinations of agents makes clinical trials more complex. Another complicating factor is timing when to give a vaccine relative to other interventions, such as surgery.
Immunity monitoring. Tracking acquired immunity is important for assessing vaccine efficacy. For cancer vaccines, new T-cell monitoring techniques are needed.
Scalability. Personalized cancer vaccines could pose logistical challenges. Streamlining production will be essential to keep costs down and availability high.
[ad_2]
Source Article Link

