[ad_1]
Surgeons operate to remove ‘black fungus’ from a person’s lung.Credit: Prakash Singh/AFP via Getty
In mid-2021, a deadly fungal disease called mucormycosis began surging in India’s crowded hospitals. Often referred to as ‘black fungus’, and caused by various common mould species, mucormycosis typically invades structures in the face and brain. But during the Indian outbreak, thousands of people in hospital — most of them undergoing steroid treatment for severe COVID-19 — developed fungal infections that festered unseen in the lungs1. “We missed a lot of pulmonary mucormycosis during COVID,” says microbiologist Arunaloke Chakrabarti, director of the Doodhadhari Burfani Hospital and Research Institute in Haridwar, India. The expertise and tests needed to diagnose the condition were frequently unavailable, Chakrabarti says, and many people died before they could be treated.
Part of Nature Outlook: Neglected tropical diseases
India’s experience with mucormycosis reflects a broader diagnostic predicament: many low- and middle-income countries (LMICs) lack even the most basic tools for detecting the fungal diseases that kill an estimated 2.5 million people each year2. Misdiagnosis often leads to incorrect treatment, and, because people who are immunocompromised are particularly at risk, expanding use of steroids and other immune-suppressing drugs is contributing to the growth of vulnerable populations. People with HIV, cancer and respiratory diseases are especially at risk, particularly where poor sanitation and over-crowding aids the spread of fungal pathogens3.
As fungal disease rates rise steadily, efforts to shore up the ability of LMICs to diagnose the conditions have taken on a new urgency. In 2022, the World Health Organization (WHO) released its first-ever ranking of fungal health threats, with the aim of strengthening the global response to infections. The report emphasized that expanded access to diagnostics will help policymakers to better assess the burden of fungal diseases, so that resources and attention can be allocated more appropriately.
A global problem
Surveys of diagnostic capacity in LMICs over the past few years reveal a dire situation. Methods in many places are limited to conventional microscopy and fungal culture, both of which have shortcomings. Microscopy, for example, requires the expertise of people who can identify fungal pathogens by their appearance. Such specialists are typically unavailable in remote settings. And it can take up to one month to culture a sufficient quantity of fungal cells for microscopic analysis. That’s too long for people who are acutely ill, such as those “with fungal sepsis or fungal meningitis, who need a more immediate diagnosis”, says Marcio Louenco Rodrigues, a mycologist at the Oswaldo Cruz Foundation, a research institute in Curtiba, Brazil. Certain pathogens, including Pneumocystis jirovecii, which causes pneumonia, can’t be cultured at all.
In the absence of a diagnosis, physicians will often treat suspected infections on the basis of symptoms. But that approach comes with risks. Some fungal pathogens, including Mucorales, Histoplasma and Aspergillus cause lethal pulmonary infections that can be easily confused with tuberculosis, which is a bacterial disease4. Fungal infections don’t respond to antibiotics. And by the time people who are initially misdiagnosed with tuberculosis receive antifungal therapy, “many are already dying”, says Claudia Banda, an infectious-disease specialist at Cayetano Heredia University in Lima, Peru.
A key step towards boosting diagnostic capacity is to broaden access to simple, point-of-care assays that can be used cheaply in remote settings. “This is what developing countries really want,” says David Denning, a research clinician at the University of Manchester, UK. Denning was the founding president of the Global Action Fund for Fungal Infections (GAFFI), a non-governmental advocacy group headquartered in Geneva, Switzerland. GAFFI played a key part in selecting tests for fungal diseases for the WHO’s list of essential diagnostics, which was first released in 2018.
Topping that list are lateral flow assays that generate fast diagnostic results and cost less than US$5 each. These sorts of assay will be familiar to many as home-test kits for COVID-19. They detect microbial antigens or immune antibodies in blood and other fluids and have already been standardized for several fungal pathogens. For example, the WHO recommends screening people with HIV who have critically low white blood cell counts for cryptococcal antigen, which would indicate the presence of a harmful fungal infection that can cause meningitis5.
Lateral flow assays detect Cryptococcus with high accuracy. But in Africa — which is home to around 65% of people in the world with HIV — only around 25% of the population has access to the test, according to a survey funded by GAFFI3. And Latin American countries have similar shortages. In 2023, Banda and her colleagues surveyed Peru’s public hospitals, and found that only 13% of them could provide cryptococcal antigen testing (unpublished data).
A push to expand access
Uptake is thwarted by a lack of awareness of fungal infections, says Rodrigues, adding that for many fungal pathogens, there are no lateral flow tests. Cost is also a barrier, because although the price per test is low, rolling them out at scale would impose substantial burdens on already stretched health-care systems.
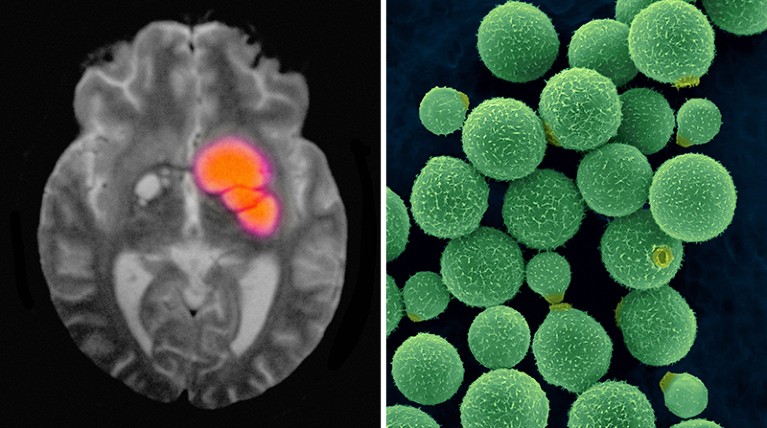
A brain scan reveals cryptococcal meningitis, which can be caused by the fungus Cryptococcus neoformans (right).Credit: Cultura Creative RF/Alamy; Dennis Kunkel Microscopy/SPL
Routine use of the assay could save many lives — Cryptococcus kills nearly 150,000 people a year2. However, Banda points out that the main drug therapy for cryptococcal meningitis, amphotericin B, is also exceedingly toxic, “so we like to have some definitive evidence of the disease before we start treatment”.
Broadening diagnostics for other fungi that can cause serious illness, such as Aspergillus and Histoplasma, could also save lives. According to Denning, as many as 10% of people with suspected tuberculosis actually have chronic pulmonary aspergillosis. “So, all tuberculosis clinics around the world should be testing for it,” he says. Denning’s research on the global incidence of fungal diseases found that more than 2 million people each year develop invasive aspergillosis2, which occurs when the fungi spreads through the body. Histoplasmosis is also endemic in many LMICs, and if left untreated, mortality in people with HIV is 100%, Banda says.
Banda’s fungal-disease work in Peru, which is funded by the US Centers for Disease Control and Prevention, shows that histoplasmosis is endemic among people with HIV, cancer and other immune-suppressing conditions who live in and around the country’s coastal jungles. Bat droppings in these areas are a ready source of the fungus spores. Accurate estimates of the number of people affected are unavailable, however, owing to the lack of access to diagnostic testing, Banda says. She cites a similar need for access for chronic pulmonary aspergillosis and Candida auris — a hospital scourge that’s notoriously difficult to eliminate or control. The fungus C. auris, in particular, is an emerging global-health threat; some strains resist all antifungals and incur death rates as high as 70%. In Peru, the pathogen emerged several years ago, “and not all our microbiology labs are adequately prepared for it”, Banda says.
Advocates are calling for lateral flow assays to be developed for a broader array of fungal diseases. Along those lines, researchers at the University of Exeter, UK, are developing an assay specific to Rhizopus arrhizus6 — a fungal spore responsible for most human cases of mucormycosis, the disease that plagued India during the COVID-19 pandemic.
But lateral flow assays can’t solve the diagnostic shortages on their own. The tests are valuable as screening indicators that reveal whether a given pathogen is — or was — present in the body, but they don’t measure the extent of a fungal infection, or “how well a patient responds to treatment”, says Amir Seyedmousavi, a clinical microbiologist at the National Institutes of Health Clinical Center, in Bethesda, Maryland. Patient monitoring requires more advanced analytical techniques, Seyedmousavi says, such as polymerase chain reaction (PCR) sequencing and other molecular tests. The laboratory capacity needed for these technologies, however, is often unavailable in resource-poor areas.
Seyedmousavi chairs the Fungal Diagnostic Working Group of the International Society for Human and Animal Mycology. The group is working to standardize and improve affordable diagnostic tests — especially for LMICs. And it aims to create international networks for knowledge sharing and education. Chakrabarti, meanwhile, is working to establish diagnostic reference centres across India that offer a range of standardized diagnostics — not just at the hospitals at which they are based, but also at the clinics that serve as the first point of contact for health care. The reference centres provide training to boost laboratory workforces, Chakrabarti says, and staff there conduct epidemiology studies to map the distribution of fungal diseases throughout the country.
During the peak of the COVID-19 pandemic, mucormycosis cases surged in India.Credit: Prakash Singh/AFP via Getty
GAFFI is sponsoring similar approaches in other parts of the world. In partnership with the non-profit organization the Asociacion de Salud Integral in Guatemala City, for instance, GAFFI sponsored a diagnostic demonstration that focused on a number of fungal diseases, including histoplasmosis and cryptococcosis. In 2023, GAFFI reported that mortality from fungal infections in people with HIV could be substantially reduced with readily available diagnostics and treatment.
Efforts to expand access to diagnostic tests face complex challenges. Because clinical awareness of fungal diseases is low in LMICs, physicians don’t request diagnostic tests often enough to create a market that could make the tests more readily available. In the absence of better information on prevalence — especially outside urban areas — health systems in low-income countries have failed to prioritize fungal diseases, Banda says, creating a vicious cycle of neglect.
Health-care financing poses extra hurdles. In some LMICs, people have to pay for fungal diagnostic tests out of their own pocket. “People don’t want to pay for a test that might be negative,” Denning says. “They just want the doctor to make a judgement about treatment. For the life-threatening fungal diseases — the ones that cause meningitis or sepsis or pneumonia — that can be tough.”
Denning argues that teaching hospitals in LMICs should have access to every test on the WHO’s list of essential fungal diagnostics, and that the tests should also be made freely available to people at the point of use. If hospitals don’t improve their capacity to detect and treat fungal diseases correctly, he warns, “then acquired microbial resistance and inappropriate use of all these antimicrobials just becomes rife”. Fortunately, diagnostic tests are steadily becoming cheaper, “so it should be possible to provide them at low-cost around the world,” Denning adds. “The big need is just to get them into everybody’s hands.”
[ad_2]
Source Article Link


