[ad_1]
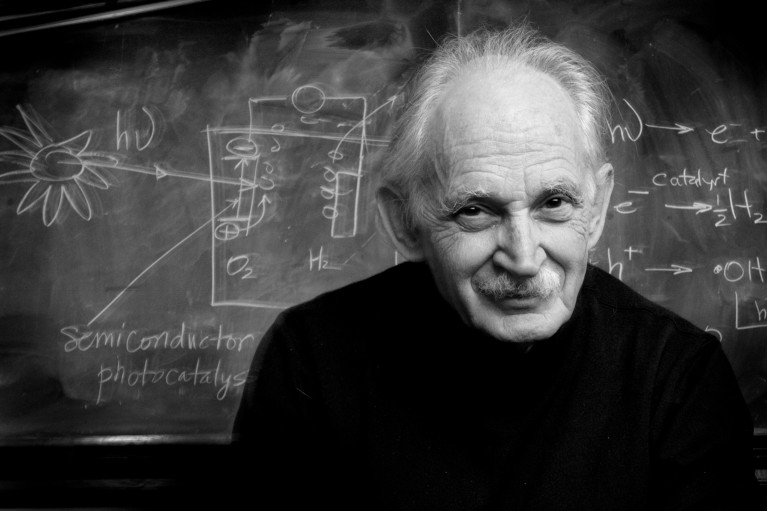
Credit: The University of Texas at Austin
Allen Bard is widely regarded as the father of modern electrochemistry. During his prolific research career, including more than 60 years at the University of Texas (UT) at Austin, Bard became a world-renowned innovator and researcher, pioneering diverse areas of electrochemistry and technologies that are widely used today.
Bard’s work on electrochemiluminescence — luminescence induced by a reaction involving the transfer of electrons — led to the commercialization of sensitive assays for biomarkers in clinical diagnostics. Bard also developed the first scanning electrochemical microscope, a tool that has proved invaluable for investigating materials for solar cells and batteries, as well as for probing cancer cells and tracking chemical reactions.
Born and raised in New York City, Bard studied chemistry at the City College of New York in 1955. He did his graduate studies (1955–58) at Harvard University in Cambridge, Massachusetts, briefly under Nobel laureate Geoffrey Wilkinson, who specialized in organometallic compounds. Bard’s presence in Wilkinson’s laboratory when the group identified the structure of ferrocene — the most ubiquitous electrochemical standard in electrochemistry — was a harbinger of great things to come.
After Wilkinson left Harvard in 1955, Bard moved to James J. Lingane’s research group, where he completed his dissertation on the electrochemistry of tin. He also worked with chemist David Geske on early attempts to apply electrochemical methods to the study of reaction mechanisms. He was introduced to the electrochemistry of aprotic solvents (unlike water, they lack an acidic proton), in which highly reactive species can be generated that would otherwise be quenched by reactions with protons.
The new car batteries that could power the electric vehicle revolution
Bard was subsequently hired as an instructor at UT Austin by Norman Hackerman, a chemist who specialized in electrochemical measurements of corrosion. In the 1960s, Bard and others established the important role of radical ions (ions that have an extra electron) in oxidation and reduction reactions of organic compounds. His group demonstrated that these species resulted from transfers of a single electron, a concept that was not generally accepted at the time. This work led Bard’s research into the area of electrogenerated chemiluminescence — in which species generated at electrodes form excited states that emit light.
Bard was a continual pioneer and rapid adapter of new electrochemical techniques. He developed many different approaches, including the rotating ring-disk electrode, used in hydrogen generation; alternating-current impedance methods for measuring fast electron transfer; and the use of digital simulations for analysing electrochemical processes. These methods provided fundamental insights into how electrons move (as a current) across interfaces and into solution as the electric potential (voltage) is varied.
From 1979 to the end of the 1990s, Bard developed the microscopic detection of electrochemical processes using piezoelectric motors, work that ultimately resulted in the development of scanning electrochemical microscopy. This technique can image electrochemical reactions on surfaces at scales from micrometres to nanometres. In collaboration with chemist Fu-Ren ‘Frank’ Fan, Bard used this form of microscopy to conduct the first electrochemical measurement of a single redox molecule, which for analytical chemists is the ultimate achievement at the limit of detection.
How to make lithium extraction cleaner, faster and cheaper — in six steps
Bard’s interests didn’t stop there. During the global oil crises of the 1970s, he was a pioneer of solar fuels — chemical energy sources produced using sunlight and stored for later use. He adapted the physics and materials science of metal–semiconductor junctions, or Schottky barriers, and applied electrochemical methods to split water molecules to release hydrogen, for example.
In the late 1970s, Bard’s group brought its techniques to the study of proteins and other biological molecules, including for processes such as the measurement of the electrochemical reduction of disulfide bonds in insulin and bovine serum albumin. This demonstrated the viability of protein electrochemistry, and such methods have since been used to study the movement of electrons in biological systems such as photosystem II and the fungal enzyme laccase in biofuel cells.
In 1980, Bard and his former PhD student Larry Faulkner penned the seminal textbook Electrochemical Methods, which will continue to inform generations of electrochemists. The latest, 3rd edition contains contributions from one of us (H.S.W.). Bard served as editor-in-chief of the Journal of the American Chemical Society from 1982 to 2001.
Bard was of the ‘old school’ of researchers and was dedicated to deep fundamental investigations of select topics. Nonetheless, he was always on the lookout for new ideas, asking colleagues: “What’s the new science here?” He prized innovation, thoroughness and independent thought.
His vast and lasting academic legacy includes more than 1,000 research papers and more than 30 patents. Perhaps the greatest legacy lies in the people that Bard worked with and mentored. Over his almost 65 years at UT Austin, Bard supervised some 90 PhD students and collaborated with around 200 postdoctoral associates and many visiting scientists.
In 2002, on his receipt of the Priestley Award — the highest award of the American Chemical Society — Bard told Chemical Engineering News: “Whatever I’ve done as a scientist will be there for a while, but then fade away. The big names in science quickly become unknown. But through your students you maintain a presence in future generations, and they go on and on and on.” In this regard, Bard’s work is enshrined in the chemistry community, scientific literature and history books.
Competing Interests
The authors declare no competing interests.
[ad_2]
Source Article Link