[ad_1]
THE PAPER IN BRIEF
• Gut bacteria can affect biological processes at body sites far from the gut.
• The extent to which gut bacteria can affect reproductive tissues is not fully understood.
• Argaw-Denboba et al.1 report that altering the community of gut bacteria in male mice had negative consequences for the health and lifespan of offspring.
• Abnormalities in sperm RNA and the placenta were some of the changes associated with changes to male gut microbes.
• More work will be needed to uncover all the mechanisms underlying this phenomenon.
LIISA VEERUS & MARTIN J. BLASER: The power of paternal bacteria
The microbial communities living in and on animal hosts have become a notable focus of scientific research in recent decades. Studies have explored the many interactions that these microbiomes have with their hosts, and the consequent implications for health and disease. Argaw-Denboba and colleagues now present work that contributes to the emerging field of cross-generational microbiome effects. They investigated how the gut microbiome of male mice might affect the health of the animals’ offspring through changes in the paternal germline tissue, which contains the cells that form sperm. The authors’ observations pointing to a gut–germline axis could, if confirmed, shift the focus of microbiome research from the current mother–newborn model2 towards a new mother–father–newborn interactive system.
After changing the community of gut microbes in prospective fathers by administering either gut-specific (primarily non-absorbable) antibiotics or laxatives, the authors showed that the sperm from a father with a perturbed gut microbiome triggered changes in the placenta (which forms from cells of the embryo) that developed in its mating partner. Some of the resulting offspring had a lower birth weight and a higher chance of premature death (Fig. 1) than did offspring of fathers with a normal microbiome.
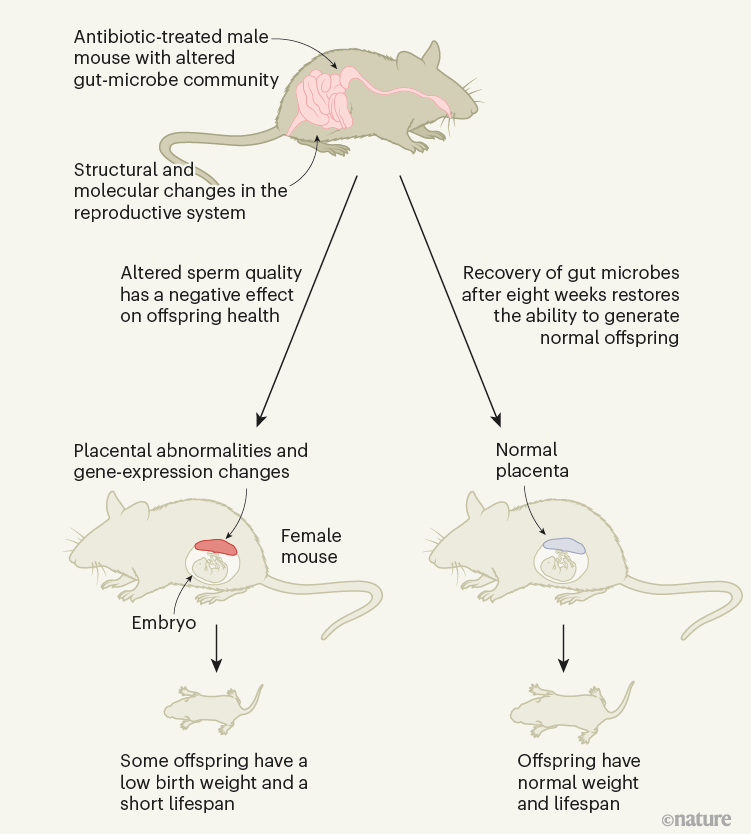
Figure 1 | The effect of male gut microbes on offspring health. Argaw-Denboba et al.1 report that using antibiotics to alter the community of gut microbes in male mice affected the production of healthy sperm in a way that had negative consequences for the development of embryonic cells into the placenta and for offspring weight and lifespan. The molecular pathways underlying this phenomenon are not yet fully understood. The effect was reversed after recovery from antibiotic treatment.
By using a variety of methods, such as microbiome transplantation, in vitro fertilization and analysis of gene expression, Argaw-Denboba et al. go beyond correlation to pinpoint that the disadvantageous effect in the offspring is transferred through sperm cells, not through the paternal microbiome. And they demonstrate that the effect is not inherited genetically, but through epigenetic modifications (alterations that do not change the DNA sequence) in the male reproductive system, with differences in sperm RNA reported. The authors also show that the paternal microbiome was restored naturally within eight weeks of the perturbation, and that this restoration was associated with a return to producing healthy offspring, indicating that the microbiome alteration effect was short-lived.
Read the paper: Paternal microbiome perturbations impact offspring fitness
One limitation of the study is that it does not define the molecular pathway through which the perturbed gut microbiome affects the male germline. Doing so could be a goal of future research. The authors note that the disadvantageous aspects of offspring development, including severe growth restriction, did not arise in all offspring, suggesting that further investigation will be required to understand the proposed gut–germline axis and its effect on offspring health.
Whether these findings in mice are also relevant to humans remains to be determined. Another interesting question is how long the gut microbiome takes to recover in people who receive a course of antibiotics. The authors’ finding that the negative effect is reversible might prove useful in providing advice on the optimal timing for fertilization, to avoid costs to the offspring.
Argaw-Denboba and colleagues’ carefully planned research demonstrates how little we still know about antibiotics’ potential effects and underlying mechanisms in relation to crucial concerns such as healthy fertilization and offspring. Exploring the modulation of the gut microbiome and the consequent effects across organ systems is a scientific frontier. Although the health implications of antibiotic use in mothers and newborns have garnered interest in previous publications3,4, the role of fathers has been mostly ignored. This study shows that the preconception microbiome has a role, and that fathers are not just gene donors, but can also, through their microbiomes, affect their offspring’s health5.
YOEL SADOVSKY & ELDIN JAŠAREVIĆ: A father’s microbes and pregnancy outcomes
In mammalian species that form placentas, embryonic development and subsequent fetal growth depend on the genetic contributions and related signals carried in the egg and the sperm, with roles for the placenta, the maternal host tissues and the external environment. These influences are mediated by the exchange of gases, nutrients and metabolic waste, and are modulated by hormones. They are also affected by exposure to microbial or viral disease-causing agents and to inflammation, toxic compounds and social and behavioural stressors. The integration of these signals determines the outcome of pregnancy, and adverse influences can compromise fetal and maternal health and lifespan.
The journey to understand previously unknown microbial genes
A key challenge in studying pregnancy relates to the dynamic and complex signals arising from factors that the parent encounters during their lifetime (described as lifetime exposures), and to how these signals affect fetal and placental development. A mother might generate or modulate health-related signals in many ways during pregnancy. By contrast, the father’s influences are limited mostly to sperm-dependent genetic (DNA) contributions, and to effects resulting from epigenetic modifications of DNA and its associated proteins, which are commonly induced by stress and dietary changes6,7. Paternal effects on offspring health, such as those mediated by stress, exposure to inhaled or ingested chemicals, or male help in providing maternal nutrition, are thought to be indirect when compared with the more direct maternal effects on the offspring.
A growing body of work demonstrates that gut bacteria and the metabolite molecules that they produce are key intermediaries between maternal lifetime exposures, pregnancy outcomes and lasting outcomes for offspring8–10. In their related findings, Argaw-Denboba and colleagues add an unexpected dimension to parental gut microbiome influences on gestational biology — namely, the effect of antibiotic-mediated disruption of the paternal microbiota on a male germline. Using mice, the authors established a strong association between a perturbed paternal gut microbiome and sex-independent restriction of fetal growth; the resultant low birth weight lingered into early adulthood and was associated with reduced survival times compared with the offspring of males who had unperturbed gut microbes.
Crucially, the effect was reversed when the paternal gut microbiome was restored to normal by ceasing antibiotic exposure, and was recapitulated through in vitro fertilization using sperm from males harbouring the perturbed gut microbiome. Furthermore, the altered paternal microbiome was associated with changes in male reproductive tissue (smaller testes and seminiferous tubules with a swollen (vacuolated) appearance and thinner than normal layers of epithelial cells). The authors observed intact genomic parental-specific expression of genes (imprints) but altered transcriptome, metabolome and methylome profiles (relating, respectively, to gene expression, production of metabolite molecules and the attachment of methyl groups to DNA); these profiles were of unknown relevance to the observed outcome.
Deciphering metabolism, one microbe at a time
Do any of these changes causally explain the prenatal and postnatal effects on the offspring? Examining samples of fetal and placental tissue, Argaw-Denboba et al. listed a series of changes in the fetal gene-expression profiles, highlighting differentially expressed genes related to lipid and metabolic processes. These changes were associated mainly with the fetal brain and brown adipose tissue. Placental analysis at embryonic days 13.5 and 18.5 revealed a smaller labyrinth (the mouse placental region that governs gas and nutrient exchange between the mother and the fetus) and impaired formation of blood vessels.
Gene-expression analysis highlighted altered expression of genes related to a metabolic process called glycolysis, to the metabolism of molecules called prolactin and steroids and to several regulators of placental development (such as the genes Hand1 and Syna). Intriguingly, some of the transcriptional changes can cause placental dysfunction. Certainly, further characterization will be crucial to determine whether effects similar to those observed in the placenta-associated condition pre-eclampsia (which involves maternal hypertension and target-organ injury and can lead to fetal or maternal illness or death) are an underlying cause of disease in this context.
These exciting observations establish a link between the paternal gut microbiota, sperm RNA content and pregnancy outcome. Although the mechanisms linking altered sperm biology with changes in the offspring and placenta and with altered gene expression remain to be unravelled, this line of investigation highlights antibiotic-mediated disruption of the paternal gut microbiota as a previously unknown mode of a sperm-mediated effect on fetal development and offspring health. Furthermore, if validated in humans, the work might indicate a potentially modifiable preconceptional contribution by the father’s microbiome to pregnancy health, which would be a pioneering concept in the biology of human pregnancy.
[ad_2]
Source Article Link